#Industry News
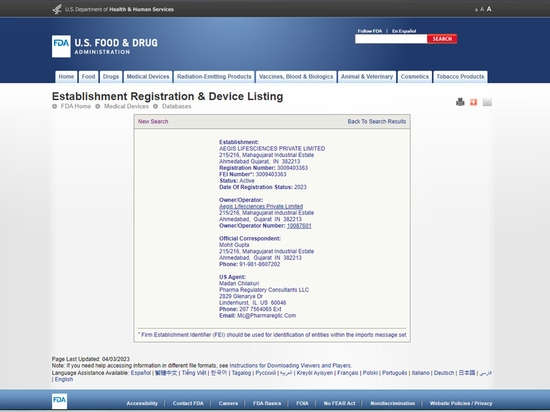
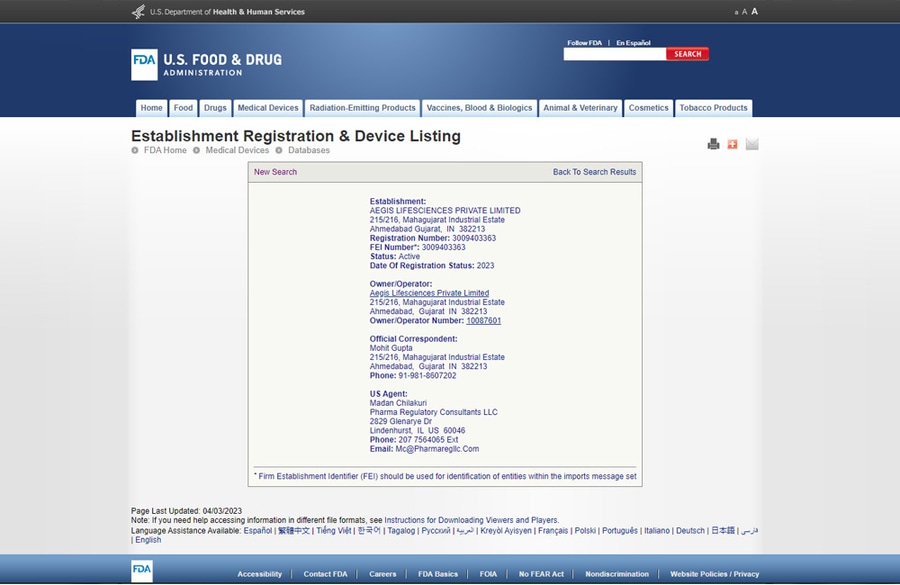
Aegis LifeSciences Pvt. Ltd. - "USFDA registered manufacturing facility"
USFDA registered manufacturing facility
As a trusted medical device manufacturer, Aegis Lifesciences Private Ltd is proud to offer a USFDA registered manufacturing facility for our absorbable haemostat products.
This registration signifies our commitment to adhering to the highest standards of quality and safety in the production of medical devices.
At Aegis Lifesciences, we are dedicated to continuous improvement and innovation. Our absorbable haemostat products are currently undergoing the 510k approval process, which will enable us to bring even more innovative solutions to the healthcare industry.
In addition to our commitment to quality and innovation, our USFDA registered manufacturing facility also provides export benefits. Many countries around the world require medical devices to meet FDA regulations in order to be sold within their borders. With our USFDA registration, we are able to export our absorbable haemostat products to a wider range of countries like; USA, Australia, Canada, Japan, Singapore, Colombia, Austria, Oman, Kuwait, Syria and Yemen. To help us to better serve the global healthcare community.
In conclusion, if you’re looking for a trusted, innovative medical device manufacturer with a USFDA registered absorbable haemostat manufacturing facility, look no further than Aegis Lifesciences Private Ltd. Visit our website quality certifications page, to learn more and stay tuned for updates on our 510k approval process