#Industry News
How Software As Medical Devices Is Revolutionizing Patient Care
Explore how digital health apps are changing the face of healthcare.
The proliferation of digital technology and changes on a global scale have made remote healthcare increasingly popular. Among the innovations we've seen in recent years are digital health apps, which open up new avenues for patient care.
Historically, medical devices have always been defined as physical tools. Stethoscopes and scalpels, needles and forceps… these objects make sense as medical devices as they make a physical impact on a patient. But personal health apps are purely software, lines of code and programming. This makes them incredibly flexible and available to a wide number of people, as they can potentially be used by anyone who owns a smartphone or needs access to that via a medical computer (for example, a primary care provider).
Today, we'll break down the rise of digital health apps, how they're used, and some of the challenges they'll have to overcome as they become increasingly popular.
Defining Software as Medical Devices
The International Medical Device Regulators Forum defines software as medical devices (SaMD) as "software intended to be used for one or medical purposes that performs these purposes without being part of a hardware medical device." In layman's terms, this means that SaMD programs function as medical devices even when installed on normal consumer products, like smartphones.
SaMD, also known as "companion apps" or "stand-alone software" are most commonly used for tracking patients or monitoring physical activity.
Common Applications for Mobile Health Apps
Remote Monitoring: Patients are typically kept confined to the hospital ward after surgery so that providers can monitor their condition. By sending patients home early and tracking their state with a mobile health app, healthcare groups can free up beds earlier and reduce patient time in the ward itself.
Telemedicine: Prior to COVID-19, telemedicine was already growing popular. However, the pandemic led to an explosion in its usage. Remote communication between providers and patients spares the latter a trip to the hospital or clinic. This is especially helpful for people living in remote or rural areas. It is also a boon to patients suffering from mobility issues or who have limited transportation options.
Personal fitness and health: One of the most common uses for mobile health apps is in tracking personal fitness and health goals. By using an app on their smartphone, consumers can track parameters like the number of steps they take in a day, how many calories they eat, and more.
Implementation of AI in SaMD
Like most of the healthcare field, mobile health apps are exploring the implementation of artificial intelligence. Given that these apps already collect data for analysis, letting generative AI analyze the data is an obvious addition.
AI is primarily used for predictive analytics, as these algorithms can process large amounts of data to provide insights for both patients and doctors. For instance, an AI can look at a patient’s health records, medical history, and current condition as recorded by their mobile health app to predict if they will get better or worse in the future.
By processing the enormous amount of data generated by a patient's biomarkers during monitoring, AI can also help providers make more informed decisions.
Growth and Regulation of SaMD
Most mobile health apps fall under the FDA's Class II category as devices that could pose a moderate risk to the patient if misused. According to GlobalData's prediction, the medical apps market will reach $12.1 billion by 2030.
With such a massive potential for growth, it only makes sense that regulators are paying close attention to the sector. The introduction of AI in healthcare has only further highlighted the need for stronger guidelines concerning the use of clinical decision support software, not just for mobile apps, but all of the healthcare industry.
Another major concern is privacy. The FTC recently issued a set of tools to help app developers determine if their product was subject to HIPAA regulations and help them design their apps to remain compliant with it.
Closing Thoughts
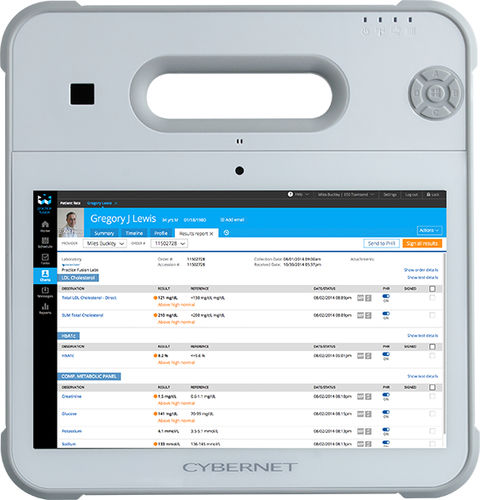
The adoption of software as medical devices and mobile health apps promises to reap benefits for both patients and healthcare providers. However, these advanced software programs will need equally-capable pieces of hardware to run on. While standard consumer devices can possibly fulfill this role, the ideal choice is a medical-grade tablet that providers can issue to their patients and securely transmit data back and forth over.
If you’re looking for a trusted medical-grade tablet manufacturer, turn to the experts at Cybernet Manufacturing. Contact our team today – we’re more than happy to explain the advantages of our expertly designed products and how they can support a variety of mobile health apps.
Join the conversation and connect with us on this and other relevant topics – Follow us on Facebook, Twitter, and LinkedIn.