#Product Trends
Fujirebio Europe announces CE marking of the fully automated Lumipulse® G SARS-CoV-2 antigen assay and the rapid antigen device ESPLINE® SARS-CoV-2
With these new products Fujirebio further positions itself as a leading global provider of immunoassay testing solutions to the IVD industry and steps up its contribution to the worldwide battle against the COVID-19 pandemic.
Gent, Belgium – August 11, 2020: Fujirebio Europe announces the CE marking of Lumipulse G SARS-CoV-2 Ag, a high-sensitivity antigen test for the fully automated CLEIA (chemiluminescent enzyme immunoassay) Lumipulse G system, and of the ESPLINE SARS-CoV-2 rapid antigen test.
With these new products Fujirebio further positions itself as a leading global provider of immunoassay testing solutions to the IVD industry and steps up its contribution to the worldwide battle against the COVID-19 pandemic. Already in May 2020, Fujirebio Europe announced the distribution and CE marking of the iAMP COVID-19 Detection kit, a molecular SARS-CoV-2 detection assay.
“We are the first company to develop and provide a fully automated SARS-CoV-2 antigen testing solution, to be used on our Lumipulse G series, together with a rapid test device on the already known ESPLINE platform; we wish and expect that these innovations will have a positive impact on result turnaround times and laboratory throughput of COVID-19 testing,” says Christiaan De Wilde, CEO at Fujirebio Europe.
About the LUMIPULSE G series
The Lumipulse G SARS-CoV-2 Ag assay will be available for use on the LUMIPULSE G1200 and LUMIPULSE G600II instruments. The LUMIPULSE G series are robust, fully automated CLEIA immunoassay instruments. The LUMIPULSE G1200 and G600II have a throughput of respectively 120 and 60 tests per hour and allow laboratory personnel to randomly load samples as needed. CLEIA technology and automated testing provides increased sensitivity, reproducibility and throughput.
About the ESPLINE series
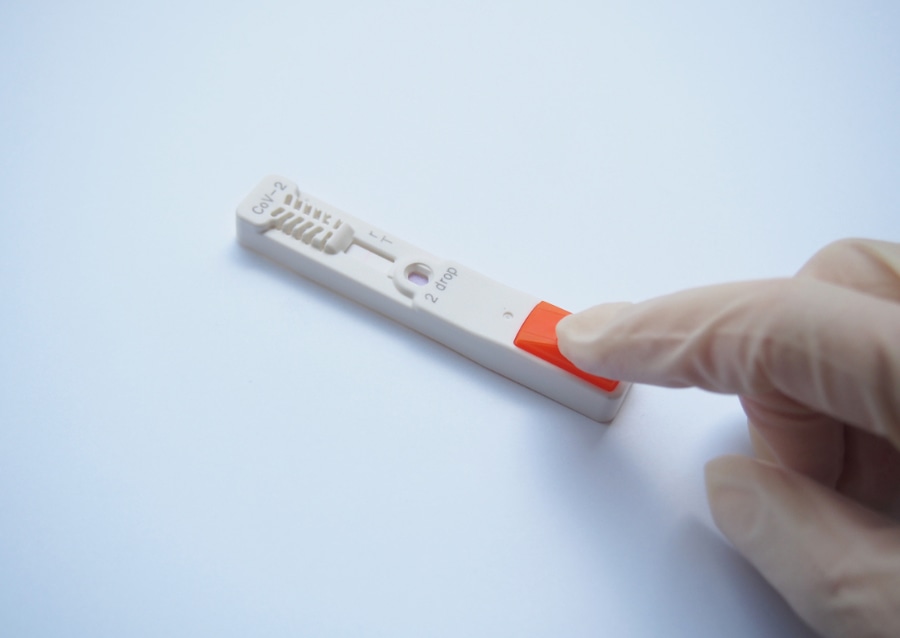
ESPLINE SARS-CoV-2 is an immunochromatographic rapid antigen test, and unlike other nucleic acid-based tests on the IVD market that also detect the SARS-CoV-2 gene with high sensitivity, the ESPLINE test does not require any special equipment. It is a cassette-style assay using a simple procedure, and it has a short reaction time (30 min).
Both assays received Japanese regulatory approval in May and June 2020 for use with nasopharyngeal and saliva samples (Lumipulse only) as an aid in the diagnosis of SARS-CoV-2 infection.
The initial submission of these tests to the FDA in the USA is expected to be made early September for nasopharyngeal swab in universal viral transport media. Submissions to expand sample types are expected to follow.
About Fujirebio
Fujirebio is a global leader in the field of high-quality in vitro diagnostics (IVD) testing. It has more than 50 years’ accumulated experience in the conception, development, production and worldwide commercialization of robust IVD products.
Founded in 1950 in Tokyo, Japan, Fujirebio has over the years concluded a number of successful acquisitions of best-in-class IVD companies. Examples include Centocor Diagnostics in 1998, CanAg Diagnostics in 2006 and Innogenetics in 2010. Today, Fujirebio’s global presence includes offices in the United States, Latin America, Europe and Asia as well as a vast international distribution network.
Fujirebio has a strong and long-lasting tradition of collaborating with experts in the worldwide clinical community in the development of high-quality routine and truly novel biomarkers that cover a variety of disease states. Its IVD product lines span the range from specialized manual and automated testing to fully automated routine clinical laboratory testing solutions.
Fujirebio is a wholly-owned subsidiary of H.U. Group Holdings Inc. (formerly known as Miraca Holdings Inc. and listed on the Tokyo Stock Exchange – TYO: 4544) and employs more than 1.200 people in Asia, Europe and America.