#Product Trends
Regulate & Measure: DBP-01P FDA Approved Blood Pressure Monitor
The U.S. Food and Drug Administration (FDA) approved the HINGMED DBP-01P fully automatic blood pressure monitor for use in the United States.
What is the FDA clearance for medical devices?
The Food and Drug Administration (FDA) is responsible for protecting public health by assuring the safety, efficacy, and security of medical devices, food supplies, drugs, biological products, and cosmetics. FDA clearance for medical devices means the product’s safety and effectiveness have been cleared by the FDA. If you want to market some class I and class II medical devices, you must file a 510(k) with the FDA. If your 510(k) submission is approved, your equipment has earned the FDA clearance, and you can legally market it. HINGMED DBP-01P kiosk blood pressure monitor gets FDA clearance and is welcomed by the majority of distributors with low MOQ.
Are blood pressure monitors FDA approved?
Before a hospital blood pressure monitor can be sold or marketed in the U.S., it must get FDA approval. Some blood pressure machines are exhibited on the market, but not approved by the authority. These kinds of machines even can bring the wrong readings for patients. It is essential to find an FDA-approved blood pressure monitor.
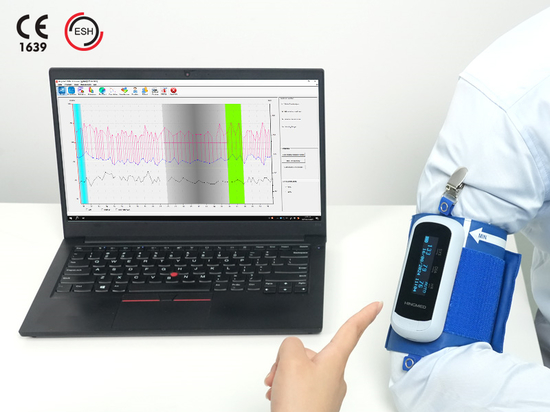
HINGMED DBP-01P FDA approved wearable blood pressure monitor
The U.S. Food and Drug Administration (FDA) approved the HINGMED DBP-01P fully automatic blood pressure monitor for use in the United States. The equipment uses the pulse wave BP motion tolerance technology to give the patients reliable and accurate measurements. The adjustable cuff can automatically change the size from 17cm to 42cm whether the patient is in a thin, middle, or fat shape. Either wearing on the left or right arm, the patient can receive the blood pressure readings around 22 seconds. The blood pressure report will be generated in a short span and improve the patient’s satisfaction. Compared to the complex maintenance, our machine can be used for 5 years. As for the repair, the staff only need to send the modular parts to the after-sales, which extremely saves time and money.
This arm-type blood pressure monitor has been sold for more than 1,512,596 units in 2023. HINGMED sincerely cooperates with distributors who have been interested in medical devices for long cooperation.