#Industry News
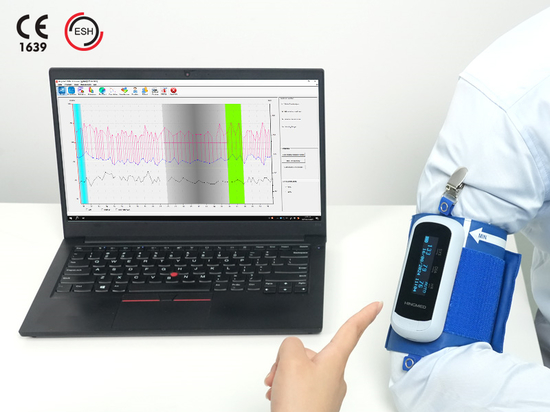
DBP-01P Clinical Automatic Blood Pressure Monitor Certified to ISO 81060-2:2018 Protocol
HINGMED DBP-01P clinical automatic blood pressure monitor is FDA approved and certified to ISO 81060-2:2018 protocol for clinical grade blood pressure accuracy.
What is the ISO 81060-2:2018 stand for sphygmomanometers?
ISO 81060-2:2018 is an international standard that specifies requirements for the safety and essential performance of non-invasive sphygmomanometers. The standard defines the requirements of accuracy and requirements of blood pressure monitors to ensure accurate and consistent blood pressure measurements.
HINGMED DBP-01P Certified to ISO 81060-2:2018 Protocol
HINGMED DBP-01P is proudly certified to ISO 81060-2:2018 protocol, ensuring accuracy and consistency in non-invasive blood pressure measurement. In light of the validation report, the validation study assesses the performance of the device DBP-01P, and HINGMED DBP-01P met the specifications required by the ISO 81060-2:2018 protocol. The evaluation of the HINGMED DBP-01P is going through rigorous tests. Before measurement, the observer is rest for 5 minutes. The machine DBP-01P is tested 3 times and observer auscultation 4 times. During the procedure, measurements were performed in the order of: auscultation, device, auscultation, device, auscultation, device, and auscultation. The observer has one minute break between measurements. Auscultation was conducted by two observers with sufficient skill at the same time with a binaural stethoscope. Through the strict clinical test, DBP-01P satisfied the ISO 81060-2:2018 protocol.
According to the conclusion, Criterion 1 was satisfied because the mean device-observer difference was < 5 mmHg, and SD was < 8 mmHg for both systolic BP and diastolic BP. Criterion 2 was also satisfied because SD was below the values reported in Table 1 of the protocol for both systolic BP and diastolic BP. DBP-01P clinical automatic blood pressure monitor enhances patient care and supports clinical decision-making.
HINGMED DBP-01P FDA Approved Blood Pressure Monitor
HINGMED blood pressure monitor factory is the first clinical automatic blood pressure monitor (including DBP-01P, and DBP-01HP) to get FDA approval. With CE, FDA approval, and adherence to rigorous safety and performance of ISO 81060-2:2018 standard, the DBP-01P sets a high standard in clinical environments, providing trusted blood pressure monitoring backed by global certification.