#Product Trends
SENSOVATION - SensoScope® Real-Time Microscopy Meets Digital Pathology Brightfield
Digital and Workflow Oriented Diagnosis
SensoScope Brightfield is an innovative digital microscopy system for pathology, combining the advantages of a conventional microscope and a modern digital scanner. The SensoScope allows live viewing of specimen and - at the same time - offers all the power of the digital world, including digitizing of full slides in several focus
levels. With this unique concept, SensoScope Brightfield follows the
workflow of the pathologists and also is an ideal tool for telepathology.
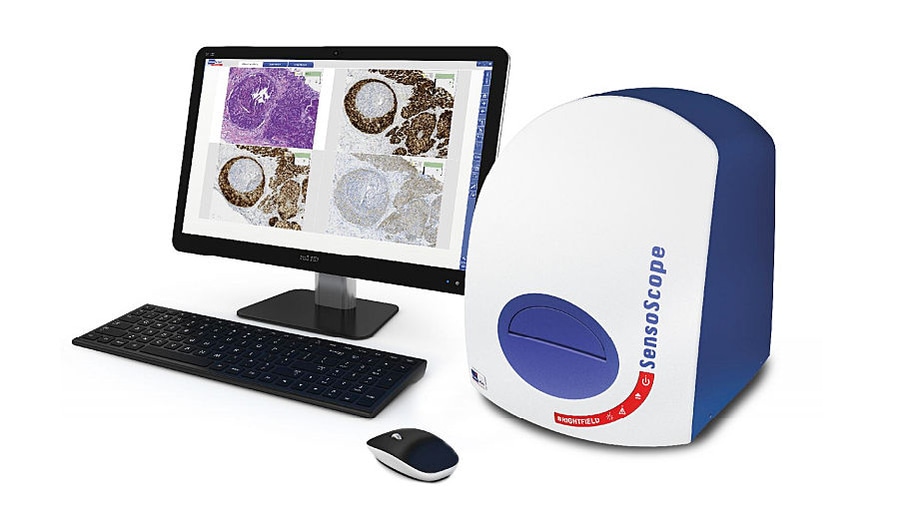
SensoScope Brightfield is a fully integrated, motorized digital microscope, ideal for visual examination, digital scanning and analysis of tissue, cells and smears on slides. High-end camera technology provides high resolution in a large field of view.
Side-by-side comparisons of specimen areas within a slide or on different slides are now possible.
Fast real-time remote control and Internet connectivity allow quick
sharing and consultation.
Effective, easy-to-use software for routine pathology and cytology
workflows enables working live with specimens and allows
automated digital scanning.
Features and Benefits
Live Microscopy Mode
- Immediate display of 4 slides simultaneously
- Ultra-rapid zoom-in and zoom-out of areas of
interest (comparable to standard microscope)
using the mouse wheel
- Up to 16 different images can be rapidly
displayed side-by-side, already stored images can be
easily added for comparison
- Snapshots of areas of interest can easily be
stored for analysis in 3rd party analysis
software
Scanning Mode
- Scan-areas can be defined manually
- Automated scanning based on profiles
- Z-Stacking up to 10 planes
Telepathology compatibility
- Real-time remote microscope control of all
functions in Live Microscopy Mode and
Scanning Mode
Magnifications:
- Standard: 2.5x, 10x, 20x, 40x
- Cyto: 1.25x, 2.5x, 5x, 10x, 20x, 40x, 63x
LIMS/HIS/LIS connectivity via Hl7 prepared
Developed and manufactured under ISO 13485
and FDA CFR 820

