#Industry News
U.S. FDA approves iRef, a manual refractometer developed for primary eye care
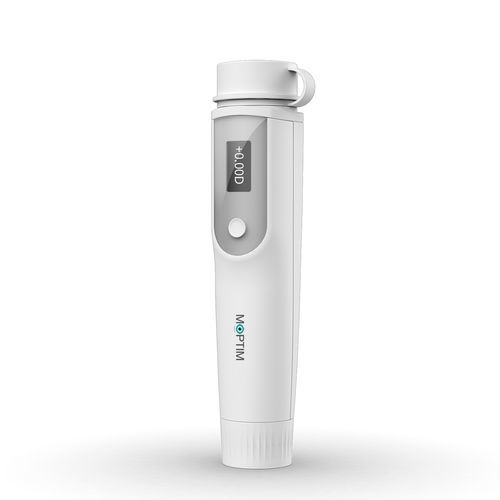
Moptim announced that it has received marketing clearance from the Food and Drug Administration (FDA), for its manual refractometer the iRef.
With this clearance, Moptim continues to deliver innovative solutions for primary eye care around the world.
iRef, which is already CE-marked approved, offers a low-cost, convenient solution for primary vision care, myopia management, vision screening, and post-operative visual acuity evaluation at home. Its compact and portable design can be used anytime, anywhere. You can also manage your eye care records and track vision changes over time through the mobile application "Eyecare".