#Industry News
More About Fiber Photometry: Working Principle and Process
This article contains more than 3,000 words to explain the working principle, and advantages, and shows the steps of setting up the relevant experiment.
A. Fiber photometry working principle.
B. Advantages of fiber photometry.
C. How to use Fiber photometry Technology?
D. Fiber photometry system setup and record a good signal.
F. Fiber photometry data analysis.
A. Fiber photometry Working principle
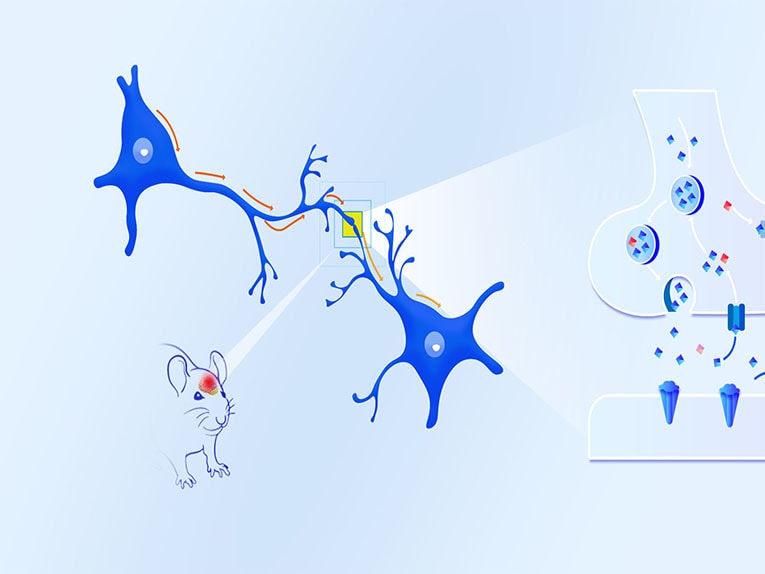
The human brain has about 90 billion neurons, which are interconnected by synapses to form a complex neural network, and thus produce various complex functions. The brain can synthesize and release hundreds of neurotransmitters, and nerve signals are transmitted between neurons through the neurotransmitters released by synapses. When the nerve excitation is transmitted to the end of the synapse, it will stimulate the calcium channel on the synapse to open and promote the influx of calcium ions, and the concentration of intracellular calcium ions will increase instantly, driving the synaptic vesicles to release neurotransmitters into the synaptic space, and that will bind to the receptors on the postsynaptic membrane and transmit the neurotransmitter signals to the next neuron, thus transmitting the information step by step
Taking the detection of calcium fluorescence signal as an example, the technical principle of Fiber photometry system is to use special fluorescent indicator or fluorescence probe with the help of the strict correspondence between the change of calcium concentration and neuronal activity. The concentration of calcium in the neuron is expressed through the fluorescence intensity and captured by the fiber photometry system, so as to achieve the purpose of detecting neuronal activity.
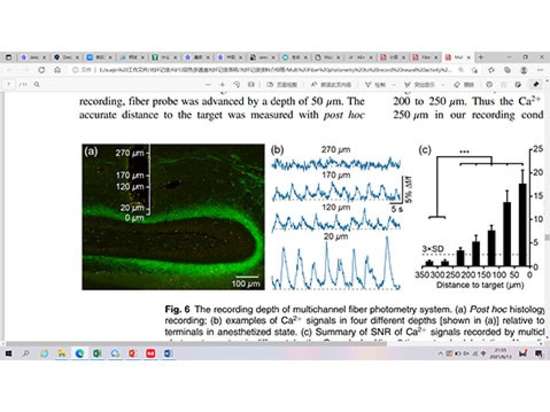
In the nervous system, the intracellular calcium concentration of neurons in resting state is 50-100nM, but when neurons are excited, the intracellular calcium concentration can increase by 10-100 times, so we can label calcium ions by injecting calcium gene coding indicators (Calcium indicator, such as GCaMPs, RCaMPs, etc.).When the neural activity is enhanced, the calcium channel opens, a large amount of calcium inflows and binds with CaM, resulting in the interaction between M13 and CaM domains, leading to cpEGFP structural rearrangement, thus enhancing the green fluorescence signal.(Figure 1) Therefore, we can characterize the activity of neurons by detecting the changes of calcium signals, and then study the correlation between neuronal activity and animal behavior, and explore the regulatory mechanism behind complex behavior.
The principle of fiber photometry for the detection of neurotransmitter signals is the same as above. A new series of genetically encodable fluorescent probes, called GRAB (GPCR activation-based), has been developed. By embedding a fluorescent protein (cpEGFP) sensitive to structural changes into the neurotransmitter receptor, the research group was able to convert the chemical signal of neurotransmitter into a fluorescent signal and, in combination with existing imaging techniques, to monitor the dynamic changes of neurotransmitter concentration in real time
B. Advantages of Fiber photometry
The study of the nervous system of awake and freely moving animals is a crucial issue in contemporary neuroscience research. By detecting calcium signals associated with neuronal activity, we are able to better understand complex neural circuits. In recent years, technological developments including two-photon microscopy, single-photon microscopy, and fiber photometry have allowed us to perform real-time detection of changes in calcium signals in animals. The application of these techniques requires engineered fluorescent proteins.
In the past, most in vivo calcium imaging was done under two-photon microscopy. Two-photon microscopy is a fluorescence imaging technique in which two beams of coherent laser light are focused through the eyepiece of a microscope to a point defined by a point spread function, similar to confocal microscopy that selectively excites fluorescent-like molecules. In two-photon microscopy, the excitation light wavelength is near 700-1000 nm, close to the infrared spectrum. This is the first advantage of in vivo two-photon imaging: longer wavelengths of light scatter less in the tissue and penetrate to greater depths. The second is the good resolution that allows visualization not only of individual neurons but also of calcium activity at the ends of axons of subcellular structures.
Laser scanning techniques in two-photon microscopy are also used to detect rapid calcium dynamics in neurons during in vivo imaging, but the excitation power to distinguish signal from noise usually needs to be high enough that this causes some degree of bleaching, and photobleaching timeouts can negatively affect image quality.