#Product Trends
Quality Control Considerations for POCT Chemistry Reagent Discs
Seamaty Reagent Discs
The rise of point-of-care testing (POCT) has revolutionized healthcare by bringing rapid diagnostics closer to patients. At the heart of this revolution are POCT chemistry reagent discs, pre-loaded with the necessary chemicals for instant analysis. While their speed and convenience are undeniable, ensuring accuracy in POCT results is also important. Improper quality control can lead to misdiagnosis, inappropriate treatment, and even patient harm. This blog post delves into the crucial considerations for ensuring the reliability of POCT testing through effective quality control practices of chemistry reagent discs.
Understanding the Risks:
Inaccurate POCT results are not merely inconvenient; they pose significant risks. Imagine a delayed diagnosis of sepsis due to a faulty cartridge, or a patient receiving unnecessary medication based on an erroneous reading. The stakes are high, highlighting the need for a proactive approach to quality control in POCT settings. Unlike controlled laboratory environments, POCT often faces unique challenges like temperature fluctuations, handling errors, and equipment issues, all of which can compromise the integrity of chemistry reagent cartridges and kits and ultimately affect test accuracy.
So, how can we ensure that the convenience of POCT doesn't come at the cost of accuracy? Here are some key quality control considerations:
Pre-Analytical Phase:
Storage and Handling: Proper storage of POCT chemistry reagent cartridges and discs is crucial. Always adhere to manufacturer recommendations regarding temperature, humidity, and expiration dates. Train personnel on safe handling practices to avoid contamination and damage to the discs.
Patient Preparation: Accurate results start with proper patient preparation. Ensure patients follow any pre-test instructions, like fasting or avoiding certain medications, to prevent erroneous test outcomes.
Equipment Maintenance: Regularly calibrate and maintain POCT equipment according to manufacturer guidelines. Faulty equipment can significantly affect test accuracy.
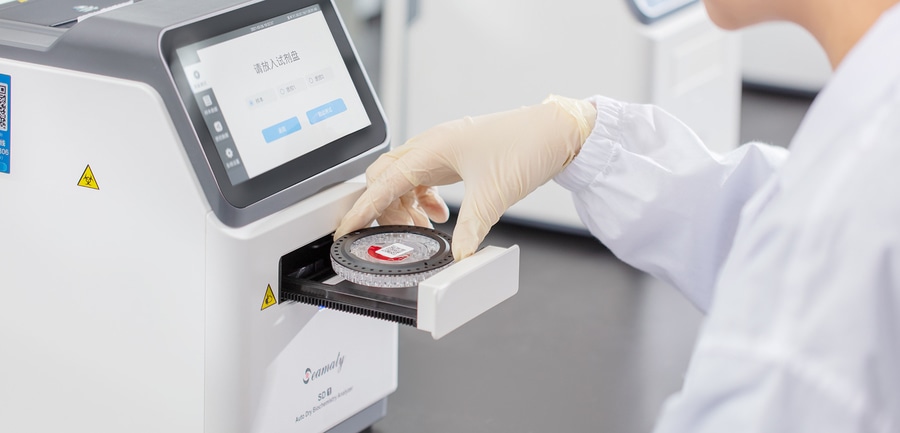
Seamaty POCT chemistry analyzers exemplify best practices in pre-analytical quality control. All Seamaty analyzers utilize dedicated chemistry reagent discs that come pre-portioned with both reagents and diluent, eliminating the need for manual mixing and potential errors. Additionally, the discs contain lyophilized reagents with a long shelf life of up to one year when stored at 2-8°C, reducing the risk of contamination and minimizing waste due to expiration.
Analytical Phase:
Internal Quality Control: Regularly run internal quality control checks using dedicated control discs specifically designed for the tests being performed. These checks verify the functionality of the reagent cartridges and discs and identify potential issues with the testing process.
External Quality Control: Participate in external quality control programs to compare your results with those of other laboratories. This provides an independent verification of test accuracy and helps identify any systematic biases or errors in your POCT system.
Documentation: Document all quality control procedures and results meticulously. This ensures traceability and facilitates investigation if any discrepancies arise.
Post-Analytical Phase:
Data Interpretation: Interpreting POCT results requires trained personnel who understand the limitations of the tests and various factors that can influence outcomes. Always consider clinical context and patient history alongside test results.
Reporting and Communication: Communicate test results clearly and promptly to healthcare providers and patients. Ensure proper documentation and follow-up as needed.
Best Practices and Tips:
Implement a comprehensive quality control program tailored to your specific POCT needs and settings.
Invest in ongoing training and education for healthcare personnel using POCT chemistry kits. Knowledge and awareness are key to maintaining accurate results.
Stay informed about emerging technologies and advancements in POCT quality control. Regularly review and update your protocols as needed.
Conclusion:
Ensuring accurate POCT testing is not simply a technical challenge; it's a critical responsibility that safeguards patient health and well-being. By prioritizing quality control measures throughout the entire testing process, from pre-analytics to post-analytics, we can leverage the power of POCT chemistry reagent cartridges and discs for the benefit of patients and healthcare professionals alike. Remember, accuracy is not a luxury in healthcare – it's a necessity. Let's work together to ensure that every POCT result delivers the reliable information it promises.