#Industry News
How to assess the progression, stability or remission of tumor status
RECIST (The Response Evaluation Criteria In Solid Tumors) is a set of definitions for assessing the effectiveness, stability, or ineffectiveness of tumor treatment.
Background
RECIST (The Response Evaluation Criteria In Solid Tumors) is a set of definitions for assessing the effectiveness, stability, or ineffectiveness of tumor treatment. This guideline mainly introduces the standardized method of objectively evaluating the changes in tumor size in clinical trials of solid tumors in adults and children. The first tumor response criteria were published by WHO in 1981, and in 2000, EORTC, NCI, and NCIC developed the new criteria, RECIST 1.0. In 2009, a revised version was released as RECIST 1.1.
Figure 1
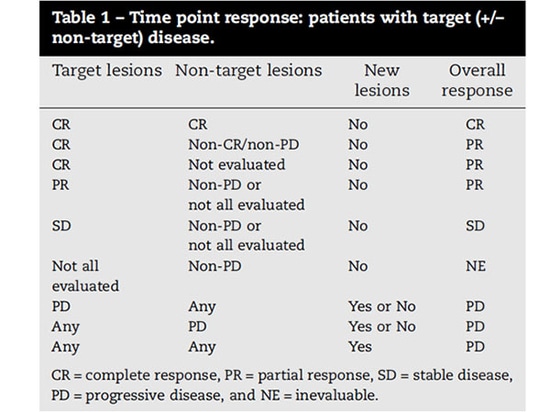
Tumor response evaluation according to RECIST 1.1[1] is divided into four categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Evaluation of tumor disease sites must use the same methods as baseline, including consistent enhancement and timely scanning.
The specific steps for tumor response evaluation are:
1.Baseline recording of target and non-target lesions.
2.Evaluation of target and non-target lesions according to the criteria.
3.Overall evaluation based on the comprehensive assessment.
Baseline recording of tumor lesions
Target lesions: Up to a maximum of 2 lesions per organ and 5 lesions in total are considered as baseline target lesions, if they are measurable.
Non-target lesions: All non-measurable disease is considered as non-target lesions. Measurable lesions that are not identified as target lesions are also included in non-target disease.
01 Measurable lesion
A lesion in which at least one dimension can be accurately measured, and the longest diameter should be recorded.
1)Evaluated by CT or MRI, the lesion should have a longest diameter of at least 10 mm (2 slice thickness, with slice thickness no greater than 5 mm).
2)Evaluated by chest X-ray, the lesion should have a longest diameter of at least 20 mm.
3)Evaluated by caliper measurement, a superficial lesion with a longest diameter ≥10 mm.
4)Malignant lymph nodes: Pathologically enlarged and measurable, a single lymph node on CT scan should have a short axis ≥15 mm (CT scan slice thickness not exceeding 5 mm is recommended). Only the short axis should be measured and followed up at baseline and follow-up.
Note: The shortest axis should be used as the diameter for malignant lymph nodes, while the longest axis should be used for other measurable lesions.
02 Non-measurable lesions
All other lesions, including small lesions (longest diameter <10mm or short axis of pathologically enlarged lymph node ≥10mm and <15mm) and truly non-measurable lesions. Lesions are considered truly non-measurable if they include:
1)Meningeal disease.
2)Ascites, pleural effusion, or pericardial effusion.
3)Inflammatory breast disease, lymphatic involvement of the skin or lungs.
4)Abdominal mass/organomegaly that cannot be measured by imaging techniques that are repeatable.
Note: Many scholars believe that after treatment, a very small amount of undetected tumor cells or tumor cell-derived molecular abnormalities may still exist, even though the lesions appear to be completely cleared by imaging. These residual tumor foci are called Minimal Residual Disease (MRD), also known as Molecular Residual Disease. MRD, as a "non-measurable lesion" on the imaging level, is receiving increasing attention and research.
03 Other special circumstances
1)Bone lesions: Bone scan, PET scan, or plain radiography are not considered as imaging techniques for measuring bone lesions. However, these techniques can be used to confirm the presence or disappearance of bone lesions.
2)Previous local treatment: Lesions previously treated with radiation therapy (or other local treatments) are considered non-measurable lesions, unless there is progression after treatment completion.
3)Normal structures: Simple cysts should not be considered as malignant lesions (neither measurable nor recorded as target lesions); normal nodules: Nodules with a short axis <10 mm are considered normal and should not be recorded or classified as measurable or non-measurable lesions.
Assessment of target lesions
Complete Response (CR): All target lesions have disappeared. Any pathological lymph nodes (whether target or non-target) must have decreased in short axis to <10 mm.
Partial Response (PR): The sum of the longest diameters of the target lesions has decreased by at least 30% compared to the baseline.
Stable Disease (SD): The decrease in the sum of the longest diameters of the target lesions does not meet the criteria for PR, and the increase does not meet the criteria for PD. The minimum sum of the longest diameters during the study should be used as a reference for SD.
Progressive Disease (PD): The sum of the longest diameters of the target lesions has increased by at least 20% compared to the smallest sum recorded since the start of the study (including baseline if that is the smallest sum). In addition to the 20% relative increase, the sum must also demonstrate an absolute increase of at least 5 mm. (Note: the appearance of one or more new lesions is also considered progression).
Figure 2
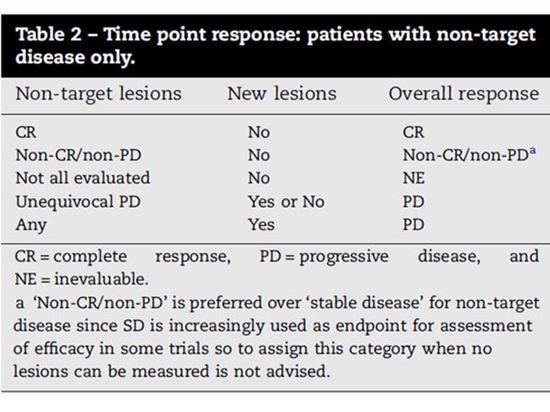
Assessment of non-target lesions
Complete Response (CR): All non-target lesions have disappeared or tumor marker levels have returned to normal. All lymph nodes must be normal in size (short axis <10 mm).
Non-PR/Non-PD: Any non-target lesion that persists and/or tumor marker levels remain above the upper limit of normal.
Progressive Disease (PD): In the presence of PR/SD of target lesions, clear progression of non-target lesions should be documented (Note: the appearance of one or more new lesions should also be considered progression).
Figure 3
New lesion(s)
The appearance of any new, unequivocal malignant lesion(s) indicates PD. If the new lesion(s) is not unequivocal or is too small to measure, repeat imaging evaluations should be continued. If repeat evaluations confirm the presence of a new lesion(s), PD should be recorded from the date of the initial evaluation. Lesions discovered in previously un-surveyed areas are considered new lesions.
Summary
Based on the above content, the assessment of PR for target lesions is not just about reduction in size, but also requires a decrease of at least 30%. Similarly, the assessment of PD for target lesions is not just about an increase in size, but also requires an increase of at least 20% and an absolute increase of at least 5mm. However, the overall evaluation also takes into account information such as non-target lesions and new lesions. Professional evaluation should ultimately rely on the opinion of the clinical physician.
References
[1].New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1)
[2].Nat Rev Clin Oncol, 2017, 14(6): 325-326.