#Product Trends
The Past and Present of PARPis
The Past and Present of PARPis
In 2014, the first PARP inhibitor Olaparib (Olaparib) was launched. This is the first marketed drug under the concept of Synthetic Lethality, which opened up a new path for tumor-targeted therapy.
PARP inhibitors and their mechanism
The full name of PARP inhibitors is polyadenosine diphosphate ribose polymerase inhibitors.
In 1922, Calvin Bridges, a geneticist working in the Morgan Laboratory of Columbia University, discovered that it would lead to the death of drosophila when two specific genes in drosophila were mutational inactivated at the same time while mutational inactivation in only one of these two genes would not cause fatal damage to drosophila. The so-called "synthetic lethality" means that for two genes in a cell, when any one of them is mutated alone or does not function, it will not cause cell death; and when both are mutated or cannot be expressed, it will lead to cell death. This principle can achieve selective killing of tumor cells without affecting normal somatic cells.
In 2005, two studies published by Nature at the same time confirmed for the first time that there is a synthetic lethal effect between PARP inhibitors and BRCA1/2 mutations.
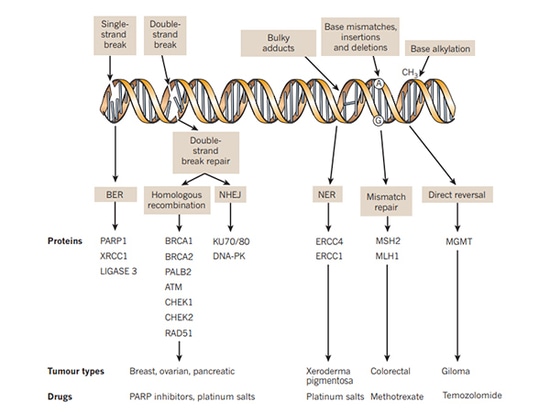
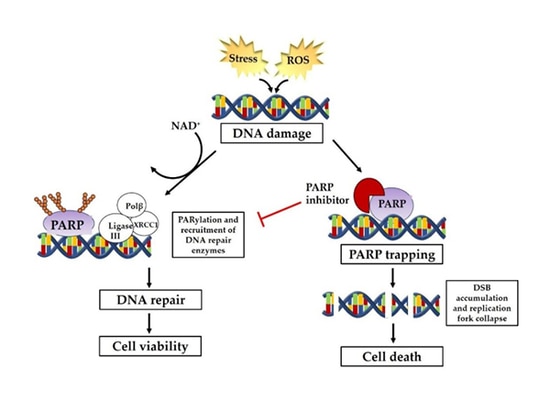
The pathways involved in DNA repair mainly include single-strand break repair (SSBR) and double-strand break repair (DSBR). The repair mechanisms mainly include homologous recombination (HR), non-homologous end joining (NHEJ), base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR) and translesion synthesis, etc. Among them, DNA single-strand repair depends on the repair pathway mediated by PARP. When single-strand DNA is damaged, PARP will gather at the DNA single-strand break site and be activated, thereby recruite a series of complexes to participate in DNA repair. PARP inhibitors inhibit PARP nzyme activity to prevent it from functioning by forming PAR polymers to attract DNA damage repair-related proteins. Homologous recombination repair (HRR) is the preferred repair method for DNA double-strand breaks. Patients with homologous recombination repair deficiency (HRD) caused by HRR gene mutations including BRCA mutations in cancer cells, germline mutations, somatic mutations of related genes repaired by homologous recombination, epigenetic inactivation and many other factors lose DNA double-strand break repair function leading to both single-strand and double-strand repair of cancer cells unable to be performed to achieve synthetic lethality.
Approved PARP inhibitors
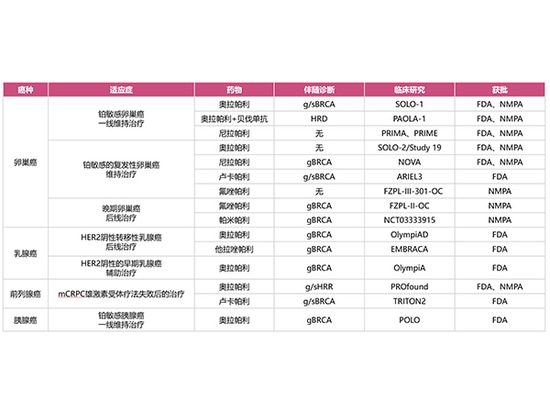
At present, the US FDA has approved 4 PARP inhibitors (Olaparib, Niraparib, Rucaparib and Talazoparib while the Chinese NMPA has approved 4 PARP inhibitors (Olaparib, Niraparib, Fluzoparib, Pamiparib), which have been applied clinically to ovarian cancer, breast cancer, pancreatic cancer, and prostate cancer.
Applicable indications approved by FDA:
Orapali:
It is used for the maintenance treatment of patients with g/s BRCA-mutated advanced ovarian cancer who achieved CR/PR after first-line platinum-based chemotherapy;
For the maintenance treatment of patients with platinum-sensitive recurrent ovarian cancer who achieved CR/PR after first-line platinum-based chemotherapy;
For the maintenance treatment combined with bevacizumab of patients with HRD-positive advanced ovarian cancer who achieved CR/PR after first-line platinum-based chemotherapy;
For the adjuvant therapy for adult patients with gBRCA-mutated HER2-negative high-risk early breast cancer who have received neoadjuvant or adjuvant chemotherapy;
For the treatment of adult patients with gBRCA-mutant, HER2-negative metastatic breast cancer receiving neoadjuvant or adjuvant chemotherapy;
For the maintenance treatment of patients with metastatic pancreatic adenocarcinoma who have not progressed on the first-line platinum-based chemotherapy regimen for at least 16 weeks and have gBRCA mutations;
For the treatment for adult patients with g/s HRR gene-mutated metastatic castration-resistant prostate cancer (mCRPC) who progressed after receiving enzalutamide or abiraterone prior to stem therapy.
Nirapali
It is used for the first-line maintenance treatment of advanced ovarian cancer patients who has achieved CR/PR with first-line platinum-based chemotherapy;
For the maintenance treatment of platinum-sensitive recurrent ovarian cancer patients
Lucapali
It is used for the maintenance treatment of patients with platinum-sensitive recurrent ovarian cancer;
For the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) who have previously received androgen receptor-targeted therapy and taxane chemotherapy and who have BRCA gene mutations.
Talazoparib
It is used for the treatment of patients with gBRCA mutation, HER2-negative locally advanced or metastatic breast cancer.
Applicable indications approved by NMPA:
Orapali
It is used for the maintenance treatment of patients with platinum-sensitive recurrent ovarian cancer;
For the first-line maintenance treatment of patients with advanced ovarian cancer with BRCA mutation;
For the first-line maintenance therapy combined with bevacizumab for patients with newly diagnosed advanced ovarian cancer who have received complete remission or partial remission after platinum-based chemotherapy and are HRD positive;
For the monotherapy for adult patients with metastatic castration-resistant prostate cancer carrying BRCA1/2 mutations (germline and/or somatic) and disease progression after previous treatment with novel hormone drugs.
Nirapali
It is used for the maintenance treatment of patients with platinum-sensitive recurrent ovarian cancer after platinum-containing chemotherapy achieves complete remission or partial remission;
For the first-line maintenance therapy for patients with advanced ovarian cancer who have a complete or partial response to first-line platinum-based chemotherapy.
Fluzoparib
It is uesd for the treatment of patients with platinum-sensitive recurrent ovarian cancer with gBRCA mutation who have received second-line or more chemotherapy;
For the maintenance treatment of patients with platinum-sensitive recurrent ovarian cancer after complete remission or partial remission of platinum-based chemotherapy.
Pamipali
It is uesd for the treatment of patients with advanced ovarian cancer, fallopian tube cancer or primary peritoneal cancer who have received at least two lines of chemotherapy and gBRCA mutation.
Applicable indications withdrew by FDA:
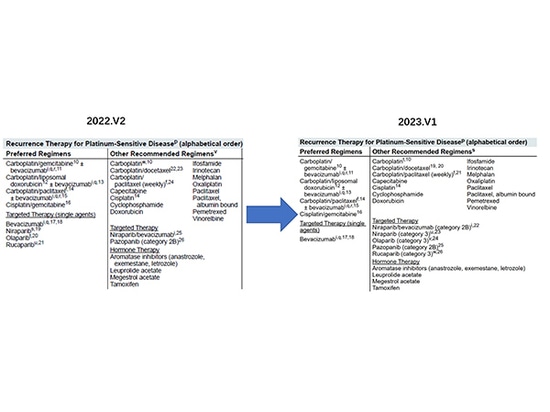
With the continuous update of clinical research, clinical data show that the risk of death of ovarian cancer patients using lucaparib, olaparib and niraparib has increased, so the FDA withdrew some of the relevant indications, of which the narrowing of indications for second-line maintenance therapy by niraparib is the most noticeable, and the NCCN guidelines have also been adjusted in time to remove the corresponding recommendation and lower the recommendation level.
1. In June 2022, the FDA withdrew rucaparib for late-line treatment for patients with BRCA-mutated ovarian cancer.
2. In August 2022, the FDA withdrew olaparib for later-line treatment of patients with advanced ovarian cancer with BRCA mutations.
3. In September 2022, the FDA withdrew niraparib for the later-line treatment for adult patients with BRCA mutations, HRD-positive and platinum-sensitive recurrent epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer.
4. In November 2022, the FDA withdrew some indications for niraparib maintenance treatment for patients with platinum-sensitive recurrent ovarian cancer.
In March 2021, the OS (overall survival) data of a NOVA study using niraparib as maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer showed that for patients with non-BRCA mutations, the median OS in the niraparib group was 31.1 months, compared with 36.5 months in the control group, which means that there is no OS benefit from niraparib maintenance therapy. In the final OS result of the NOVA study, the OS hazard ratio (HR) for non-BRCA mutation patients was 1.10 (95% CI: 0.831–1.459).
In September 2022, the American Society of Clinical Oncology (ASCO) launched "PARP Inhibitor Ovarian Cancer Management: ASCO Guideline Rapid Recommendation Update", which emphasized that for patients with non-BRCA-mutated recurrent ovarian cancer, niraparib maintenance therapy needs to be weighed between the potential PFS (progression-free survival) benefit and OS decline.
Now, with the withdrawal of the partial indications of PARP inhibitors, the clinical use of PARP inhibitors is relatively calm and there is also a demand for precise guidance of medication.
Summary
PARP inhibitors are targeted drugs that exert anti-tumor effects through the "synthetic lethal" effect on the basis of HRD. Its advent has changed the treatment model of ovarian cancer, making maintenance therapy an important part of the overall management of ovarian cancer, which has a milestone meaning.
In clinical practice, the indications for the use of PARP inhibitors should be strictly grasped. It is recommended to routinely detect BRCA gene mutations before medication, and HRD detection should be performed if conditions permit, so as to accurately guide clinical medication and evaluate prognosis.
Among the indications of PARP inhibitors, in addition to breast cancer, ovarian cancer, fallopian tube cancer, etc., prostate cancer, which is also a reproductive cancer, is also with a high research and development enthusiasm, and combination therapy is also a major research hotspot. In the future, as more clinical trials come to an end, the clinical application scenarios of PARP inhibitors and related molecular markers will become more and more clear.
references:
1. Guidelines for Clinical Application of PARP Inhibitors in Ovarian Cancer (2022 Edition)
2. Nature. 2012 Jan 18;481(7381):287-94.
3. J Clin Med. 2019 Mar 30;8(4):435.
4. FDA official website
5. NMPA official website
6. NCCN Guidelines