#Product Trends
The Application Value of Lung Cancer Methylation in Early Lung Cancer Diagnosis
The prospect of lung cancer methylation detection.
According to statistics from the World Health Organization, lung cancer is the leading cause of cancer deaths worldwide, with an incidence rate of 11.4% and a mortality rate of 18.0%. In China, lung cancer has the highest incidence rate among malignant tumors. Lung adenocarcinoma (LUAD) is the most common histological subtype of non-small cell lung cancer (NSCLC), accounting for about 40% of lung cancer cases. Surgical resection of early-stage NSCLC provides good prognosis, with a 5-year survival rate of 70-90% (stage I), but most patients (about 75%) are diagnosed at a late stage (stage III/IV) and have a low survival rate. With the development of sequencing technologies, abnormal DNA methylation patterns have been discovered in various tumors and are considered an important cause of cancer. Methylation is usually present in highly and moderately repetitive DNA sequences and plays a key role in chromosomal instability. High methylation of promoter regions of tumor suppressor genes is often associated with gene silencing. DNA methylation is involved in early stages of tumor formation. In addition, DNA methylation is relatively stable over time and can be non-invasively detected in blood, urine, saliva, and other body fluids. Therefore, an increasing number of methylation biomarkers have been developed for early screening and diagnosis of tumors. Domestic studies[1] have shown that combined methylation detection of SHOX2 and RASSF1A in bronchoalveolar lavage fluid has a significantly higher overall diagnostic efficiency for lung cancer than cytological examination and serum biomarker carcinoembryonic antigen. Another study showed that when the two genes were detected by methylation-specific PCR, the detection rates of lung adenocarcinoma and squamous cell carcinoma in individuals with positive results were 66.0% and 90.9%, respectively. This indicates the significant value of SHOX2 and RASSF1A methylation detection in early diagnosis of lung cancer.
The function and associated diseases of RASSF1A gene
The RAS-association domain family (RASSF) consists of 10 members, from RASSF1 to RASSF10. These proteins are characterized by the RAS-association (RA) domain, which can be found at the C-terminus (e.g., RASSF16, known as C-RASSF protein) or N-terminus (e.g., RASSF7-10, known as N-RASSF proteins). RASSF1 and RASSF5 are widely and significantly studied members of this protein family, while little data is available on other members.
RASSF1A and RASSF1C are widely expressed in normal tissues and are located on microtubules, participating in growth regulation. Their roles in cancer are almost opposite, with the former playing a tumor-suppressive role and the latter playing a crucial role in carcinogenesis. The transcriptional silencing of RASSF1A by high methylation disrupts the balance between the two, leading to overexpression of RASSF1C and guiding its function in cancer development. According to relevant data, RASSF1A promoter methylation occurs at a frequency of up to 88% in lung cancer, while it is almost unmethylated in normal surrounding tissue. The frequency of RASSF1A promoter methylation reaches almost 100% in small cell lung cancer (SCLC) and 65% in non-small cell lung cancer (NSCLC).
The function and associated diseases of SHOX2 gene
The SHOX protein is mainly distributed in the limbs, heart, nose, gill arches, nervous system, and mid-to-distal part of the human embryonic genital tubercle. SHOX2 is relatively close to the limbs. In addition, SHOX2 is also expressed in the nasal placode, central nervous system substrate, dorsal root ganglia, heart inflow tract, third pharyngeal arch, and derived structures. The SHOX gene mainly regulates the development of early embryonic body structures and the cardiac pacemaking system. Mutations in the SHOX gene typically lead to expression loss and may result in the syndrome of short stature.
SHOX2 inhibits apoptosis and activates the NF-kB pathway by increasing RUNX2 to suppress p53 activity, thereby leading to the occurrence of carcinogenic processes.
RASSF1A is a key regulatory factor in the Hippo pathway and links this pathway to TNF-α, NF-kB, and TGF-β signaling pathways. In cooperation with inflammatory cytokines, P53, and K-RAS, the epigenetic loss of RASSF1A links a series of important cancer signaling pathways and plays a significant role in cancer occurrence and metastasis. Due to its complex relationship with certain microRNAs, SHOX2 may interact with the Hippo, EMT, RAS/ERK MAPK, and PI3K/AKT pathways, which are critical for tumorigenesis,metastasis, and lung cancer occurrence.
SHOX2 can bind to TGF receptors and interact with the NF-kB pathway, indicating a mutual regulatory relationship with RASSF1A.[2]
Sensitivity and specificity of SHOX2 and RASSF1A methylation in clinical studies
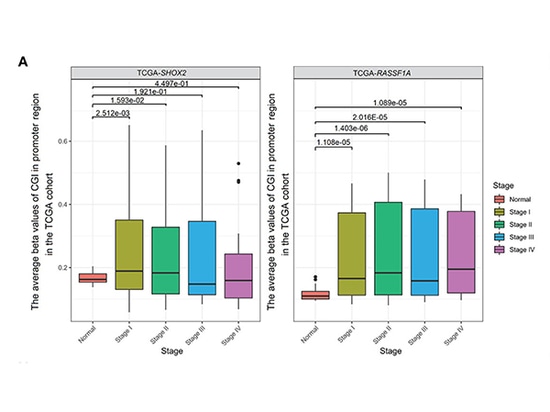
Combined SHOX2 and RASSF1A promoter methylation was determined in samples from 54 early-stage LUAD patients and 31 patients with benign lung nodules at Nanjing Drum Tower Hospital. The results showed that the detection level of SHOX2 promoter methylation in tumor samples from LUAD patients was slightly higher than that of RASSF1A. It was found that SHOX2 or RASSF1A promoter methylation detection was sensitive and specific for early-stage LUAD, but the diagnostic efficiency of single-gene methylation detection was not high. The diagnostic potential of combined promoter methylation detection for stage 0 patients is still unclear. As the disease stage increased from stage 0-II or as LUAD progressed from AIS (adenocarcinoma in situ) to MIA (minimally invasive adenocarcinoma) and then to IPA (invasive adenocarcinoma), the levels of SHOX2 or RASSF1A promoter methylation gradually increased. Patients with positive results in combined methylation detection may have rapidly progressing tumors and require active treatment. In addition, analysis of the TCGA cohort showed that SHOX2 did not significantly increase from stage III to stage IV. This may indicate that SHOX2 promoter hypermethylation is a biomarker for early-stage LUAD but not for late-stage LUAD[3].
Comparison of methylation between normal and tumor samples of stages I-IV in the TCGA cohort
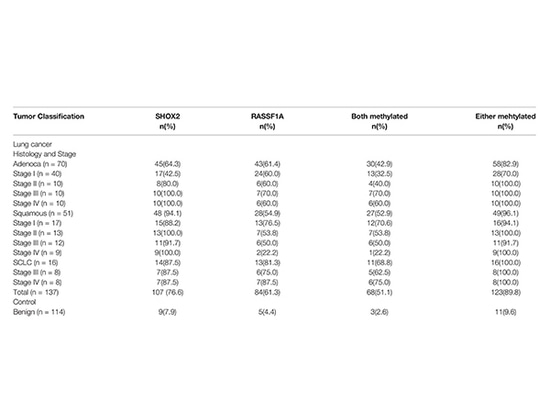
SHOX2 and RASSF1A methylation levels were detected in FFPE resection specimens from 251 patients at Tongji Hospital, Tongji University. Among them, 137 cases were diagnosed with lung cancer, including 70 adenocarcinomas, 51 squamous cell carcinomas, and 16 small cell lung cancers. Another 114 cases were benign diseases as controls. In addition, 17 biopsy specimens from lung cancer patients were analyzed. For all samples, clinical characteristics related to histological classification and tumor stage confirmed by pathology were analyzed.
Methylation of SHOX2 and RASSF1A in different histological subtypes and tumor staging groups
The positive detection rates of SHOX2 and RASSF1A were 100.0%, 96.1%, 82.9%, and 89.8%, respectively, in small cell lung cancer, squamous cell carcinoma, adenocarcinoma, and all cancers. In the control group, 11 of 114 patients with benign lung diseases were detected to be positive for SHOX2 or RASSF1A, with a specificity of 90.4%. The sensitivity of SCC detection was 94.1% for SHOX2 alone. Combined with RASSF1A, the detection rates for adenocarcinoma and small cell lung cancer were increased from 64.3% and 87.5% to 82.9% and 100%, respectively. Lower sensitivity of SHOX2 was observed only in stage I adenocarcinoma. The study also found that the degree of SHOX2 methylation was related to the staging of lung cancer, increasing from stage I to IV, while no such correlation was observed for RASSF1A methylation in lung cancer cases. In addition, highly methylated SHOX2 or RASSF1A was detected not only in 16 surgical specimens but also in 11 histopathologically negative biopsy specimens among 17 biopsy specimens. This indicates that methylation biomarkers have higher sensitivity compared to pathological results and can significantly improve diagnostic efficiency[4].
Potential of Methylation detection for early diagnosis of lung cancer
Currently, routine pathological diagnosis largely belongs to "empirical science" and is highly subjective. Pathological diagnosis (including cytological and histopathological examination) may be severely affected by specimen quality and the diagnostic level of pathologists. The sensitivity and reproducibility of detection need to be improved and enhanced. Emerging molecular diagnostic methods are more sensitive and objective and can overcome the inherent deficiencies of morphological diagnosis. DNA methylation changes are one of the most promising biomarkers for early cancer diagnosis, and have been transferred from scientific research to clinical applications. Many studies have reported various DNA methylation biomarkers and confirmed their roles in lung cancer diagnosis. The combined detection of RASSF1A and SHOX2 methylation, with its high sensitivity and specificity, may provide unparalleled biomarkers for lung cancer screening and progression monitoring. Further understanding of the biological functions of RASSF1A and SHOX2 through research and improvement of their detection methods can help better utilize them in clinical practice. The application of these biomarkers is expected to improve the accuracy of lung cancer patient screening, thereby increasing the 5-year survival rate of lung cancer patients and guiding clinical individualized treatment in a timely manner.
References:
1.Chinese expert consensus on diagnosis of early lung cancer (2023 Edition)
2 J Cancer Res Clin Oncol. 2020 Jun;146(6):1379-1393
3.Front Oncol. 2022 Jun 28;12:849024.
4.Front Oncol. 2020 Dec 14;10:565780.