#Product Trends
New Therapeutic Targets for Epithelial Ovarian Cancer
Ovarian cancer poses a significant threat to women's health.
Globally, it ranks as the eighth most common malignant tumor among women and is the most deadly gynecologic malignancy, with the highest mortality rate among the three major gynecologic tumors (ovarian, cervical, and endometrial cancers) [1]. In China, the situation regarding the prevention and treatment of ovarian cancer is equally grim. Ovarian cancer ranks third among the three major gynecologic tumors in terms of incidence and has been increasing annually. The mortality rate is also the highest among female reproductive tract malignancies [2]. Ovarian cancer is often referred to as the "silent killer" because it is located deep within the pelvis, and its early symptoms are often concealed. By the time it is diagnosed, 70% of patients are already in an advanced stage with pelvic and abdominal spread (stage III/IV) [3]. Among the various types of ovarian cancer, epithelial carcinoma is the most common, accounting for approximately 80% of malignant ovarian tumors [4]. According to the 2014 WHO classification, epithelial ovarian cancer can be further classified into serous carcinoma (70-80%), endometrioid carcinoma (10%), clear cell carcinoma (10%), mucinous carcinoma (3%), and other rare types (<5%) [5].
Serous carcinoma can be further divided into high-grade serous carcinoma (HGSC) and low-grade serous carcinoma (LGSC). HGSC accounts for approximately 70% of epithelial ovarian cancer cases, while LGSC accounts for 5% [5]. HGSC is the most common type of ovarian cancer and is characterized by strong invasiveness and poor prognosis. About 70% of patients experience recurrence within three years after standard treatment (surgery + chemotherapy), and ultimately, many are incurable due to acquired drug resistance [6]. In recent years, the prognosis of HGSC in patients with BRCA1/2 mutations has significantly improved with the use of PARP inhibitors. With the continuous elucidation of the molecular characteristics of ovarian cancer, the treatment has gradually shifted towards individualized precision therapy, and the development and application of targeted drugs have brought new hope and changed the traditional treatment approach.
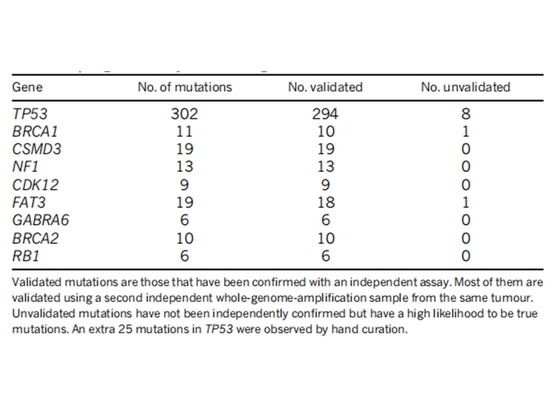
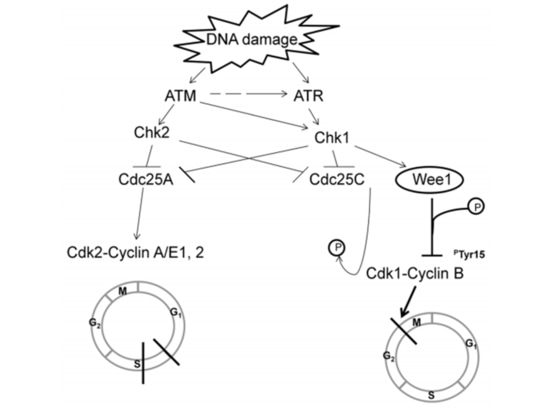
The TCGA research team analyzed the mRNA expression, microRNA expression, promoter methylation, DNA copy number, and exon DNA sequencing of encoding genes in 489 cases of high-grade serous ovarian adenocarcinoma. They found that almost all high-grade serous ovarian cancer patients had TP53 mutations (96%), followed by BRCA1/2 mutations (accumulated in germline and somatic mutations, accounting for 22% of cases) [7] (Figure 1). After TP53 mutations occur, the G1/S checkpoint function of tumor cells is lost. WEE1 kinase can block cell entry into mitosis at the G2/M checkpoint and repair damaged DNA to maintain genomic stability [8] (Figure 2). If the G2/M cell checkpoint is relieved using a WEE1 inhibitor, tumor cells' DNA damage cannot be repaired, leading to "synthetic lethality" [8]. Therefore, WEE1 inhibitors have become potential targeted therapeutic drugs for TP53-mutated epithelial ovarian cancer. Currently, only adavosertib (AZD1775/MK1775) is under clinical trial.
Recently, the results of a phase II clinical trial (NCT02151292), published in The Lancet, have attracted global attention. The study included 99 patients with advanced, refractory, high-grade serous ovarian cancer (i.e., patients resistant or refractory to platinum-based chemotherapy).
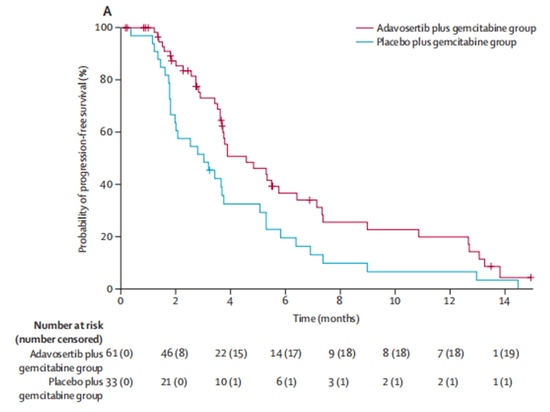
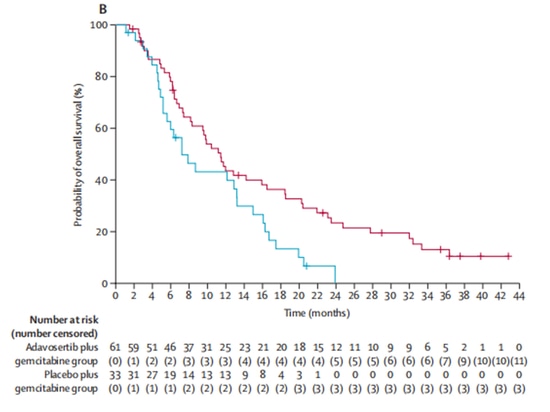
In a 2:1 ratio, the experimental group (n=65) received the investigational drug Wee1 inhibitor adavosertib in combination with gemcitabine treatment, while the control group (n=34) received gemcitabine alone. After enrollment, 5 patients dropped out of the clinical trial due to other reasons and did not receive treatment. Out of the remaining 94 patients who received treatment, the objective response rates were 23% in the experimental group and 6% in the control group, indicating a significant improvement in objective response rates with adavosertib. In terms of survival, adavosertib also significantly prolonged the median progression-free survival (mPFS) from 3.0 months to 4.6 months, with a 45% reduction in the risk of disease progression. The median overall survival (mOS) increased from 7.2 months to 11.4 months, resulting in an average extension of 4.2 months [9] (Figure 3, Figure 4). The study demonstrates clinical benefits of Wee1 inhibitor in combination with chemotherapy.
In addition, a randomized double-blind Phase II trial (NCT01357161) demonstrated that adavosertib can enhance the therapeutic efficacy of platinum-sensitive ovarian cancer with p53 mutation when combined with carboplatin/paclitaxel chemotherapy, improving progression-free survival (PFS) (7.9 months vs. 7.3 months). Adavosertib was observed to have moderate clinical benefits [10]. Another Phase II clinical trial (NCT01164995) indicated that adavosertib enhances the effectiveness of carboplatin in p53 mutant ovarian cancer, with an overall response rate of 43%, but resistance eventually develops [11]. A Phase II clinical study (NCT02272790) showed clinical benefits of adavosertib in combination with carboplatin therapy; however, this combination treatment more frequently caused hematologic toxicity compared to carboplatin monotherapy [12]. Therefore, future research is needed to optimize the treatment dosage and regimen for the combination of adavosertib and carboplatin.
PARP inhibitors based on the principle of "synthetic lethality" have demonstrated significant efficacy in the treatment of ovarian cancer. Similarly, WEE1 inhibitors based on the same principle have become an attractive option for epithelial ovarian cancer and have shown some efficacy in other solid tumors such as endometrial cancer, head and neck squamous cell carcinoma, small cell lung cancer, and pancreatic cancer. The mechanism of sensitivity to WEE1 inhibitors in ovarian cancer is also being continuously studied. Research has indicated that the activation status of mTOR significantly affects the sensitivity of cancer cells to WEE1 inhibition. It has also been reported that cancer with H3K36me3 defects is highly sensitive to WEE1 inhibition. A team led by Pan Chaoyun, Yao Shuzhong, and Wang Wei from Sun Yat-sen University in China discovered that the ODF2L gene is a key factor determining the sensitivity of epithelial ovarian tumor cells to WEE1 targeted inhibition. The combination of targeting ODF2L and WEE1 exhibits synthetic lethality [13].
The mutant p53 protein lacks suitable pockets for compound binding due to its smooth surface, posing a significant challenge in drug development. This characteristic has made TP53 one of the three major "undruggable" targets, alongside RAS and MYC. However, currently, there are two drugs on the market targeting RAS, which challenges the notion of its "undruggable" nature. There is hope that the next drug based on the principle of synthetic lethality can be approved and made available to benefit more patients.
References:
[1] CA Cancer J Clin, 2021.
[2] Journal of the National Cancer Center, 2022, 2(1).
[3] NCCN Ovarian Cancer 2023 V1.
[4] Ovarian Cancer Diagnosis and Treatment Guidelines (2022 edition).
[5] White Paper on the Diagnosis and Treatment Status of Ovarian Cancer in China (2022 edition).
[6] Advances in the Application of Precision Medicine in High-grade Serous Adenocarcinoma of the Ovary. Fudan Journal of Medical Sciences (Medical Edition), 1-10.
[7] Nature, 2011, 474(7353): 609-615.
[8] Cell cycle, 2013, 12(19): 3159-3164.
[9] Lancet, 2021 Jan 23; 397(10271): 281-292.
[10] Clinical Cancer Research, 2020, 26(18): 4767-4776.
[11] Journal of Clinical Oncology, 2016, 34(36): 4354-4361.
[12] Journal of Clinical Oncology, 2019, 37(15): S5513 [2021-10-24].
[13] Journal of Clinical Investigation, 2023; 133(2): e161544.