#Product Trends
The Biomarkers of Subtypes in Endometrial Cancer
Molecular subtyping of endometrial cancer (EC) has become a hot topic in the field of gynecologic oncology in the past couple of years.
The classification of EC has evolved from a simple histological classification to a molecular genetic classification. Through high-throughput sequencing (NGS) and other detection methods, EC has been categorized into four molecular subtypes with significant differences. The essential markers for this classification include POLE, MMR/MSI, and TP53/p53. In the Cancer Genome Atlas (TCGA) study, in addition to the aforementioned subtype markers, there are also many frequently mutated genes, such as PTEN, KRAS, PIK3CA, CTNNB1, etc. As research progresses, the roles of these supplementary subtype markers are continuously being unveiled.
TCGA Molecular Subtype Characteristics [1-2]
In addition to the supplementary subtyping, 67% to 91% of EC patients have at least one genetic mutation that could potentially serve as a target for FDA-approved drugs or drugs in clinical trial stages. Currently, there are several pan-cancer clinical studies investigating the effectiveness of targeted therapies on genes such as PI3K/ AKT/mTOR, KRAS, AKT1, FGFR2, FBXW7, and PTEN[3].
01 CTNNB1
CTNNB1 is likely one of the most extensively studied supplementary subtype genes. This gene encodes beta-catenin, which plays a role in regulating cell adhesion and cell signaling[4]. The mutation frequency of the CTNNB1 gene in the CN-L subtype is 52%[1]. Some early studies found that in G1/2 and I/II stage EC patients, despite having low rates of lymphovascular space invasion (LVSI) and muscle infiltration, pathogenic mutations on exon 3 of the CTNNB1 gene were associated with poorer recurrence-free survival (RFS) with a hazard ratio (HR) of 5.97. Tumors with CTNNB1 mutations also showed a significantly reduced likelihood of having mutations in KRAS, TP53, and FGFR2[5].
In 2016, Stelloo et al., building upon the ProMisE classification, combined CTNNB1 exon 3 mutations, high L1CAM expression (>10%), and LVSI to further propose the Trans-PORTEC classification (favorable, intermediate, and unfavorable). This classification aimed to provide a more precise risk stratification for the largest and most heterogeneous subtype of endometrial cancer, known as Non-Specific Molecular Profile (NSMP) type. It was found that the prognosis was worse for CTNNB1 exon 3 mutation subtype compared to the wild-type subtype[6]. The PORTEC-4a trial (NCT03469674) is the world's first clinical study that determines adjuvant treatment strategies based on molecular subtyping. The study aims to compare the efficacy of adjuvant treatment based on comprehensive risk analysis from molecular subtyping with standard vaginal brachytherapy for endometrial cancer. The stratification method employed is the aforementioned Trans-PORTEC classification[7].
▲Trans-PORTEC Classification[8]
In addition to the auxiliary subtyping, CTNNB1 gene mutations may also provide indications for treatment effectiveness. A Phase II study (NCT01068249) demonstrated that patients with EC harboring CTNNB1 mutations can benefit from the combination of everolimus and letrozole[9]. This treatment approach is also a preferred option for hormone therapy in recurrent or metastatic EC according to the National Comprehensive Cancer Network (NCCN) guidelines[10].
Regarding the detection methods for CTNNB1 mutations, while immunohistochemistry (IHC) for nuclear beta-catenin expression might serve as an alternative for detecting CTNNB1 exon 3 mutations, NGS testing remains the gold standard[4].
02 PTEN
PTEN is a tumor suppressor gene. The PTEN protein possesses both protein phosphatase and lipid phosphatase activities, participating in the regulation of cell cycle, proliferation, and DNA repair by inhibiting the PI3K/AKT signaling pathway. Furthermore, the loss of PTEN impairs the function of CHK1 protein, leading to the accumulation of DNA double-strand breaks and genomic instability. It also regulates the expression of RAD51, which is a key protein in homologous recombination (HR) repair[11].
▲Primary Functions of PTEN [12]
PTEN gene mutations are the most common somatic mutations in endometrioid endometrial carcinoma (EEC), representing early yet not fully established events in tumorigenesis[8]. High mutation frequencies are observed in the POLE hypermutated subtype, MSI-H high mutation subtype, and CN-L subtype, with rates of 94%, 89%, and 77% respectively. In the CN-H subtype, the mutation frequency of PTEN is only 11% to 15%, and PTEN gene mutations are rare in serous carcinoma (2%)[1]. In EEC, PTEN mutations often co-occur with PIK3CA and PIK3R1 mutations, and the loss of PTEN also exhibits synergistic effects with CTNNB1 mutations or MLH1 inactivation[7]. In most EEC cases, PTEN mutations and TP53 mutations are often mutually exclusive. However, about 50% of high-grade EEC cases with TP53 mutations have co-existing PTEN mutations, where both TP53 and PTEN mutations are non-silent[1]. Co-occurrence of PTEN mutations and TP53 mutations is common in carcinosarcomas[7]. These data suggest that while PTEN gene mutation cannot be used as a classification criterion, it can serve as a validation for subtype results.
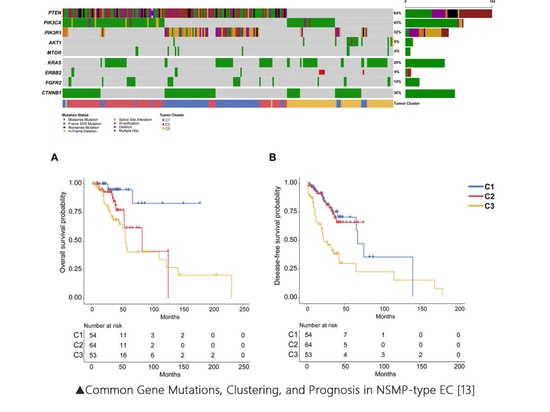
Some studies have suggested dividing the NSMP (Non-Specific Molecular Profile) type of EC into three subclusters based on the mutation status of genes like PTEN. These subclusters are as follows: C1: PTEN-PIK3R1 co-mutations, C2: PTEN-PIK3CA co-mutations, and C3: PTEN wild type with chromosome 1q copy number gain. These three subclusters exhibit significant differences in overall survival (OS) and disease-free survival (DFS)[13].
▲Common Gene Mutations, Clustering, and Prognosis in NSMP-type EC [13]
Based on the primary functions of the PTEN protein, PTEN gene mutations might be related to the efficacy of inhibitors targeting the PI3K/AKT/mTOR signaling pathway[8]. PTEN is also considered a predictive marker for sensitivity to poly(ADP-ribose) polymerase (PARP) inhibitors. There have been reports of advanced EC patients with multi-organ metastases (liver, lung, brain, etc.) who are BRCA-negative and have PTEN loss. These patients survived for 10 months after treatment with olaparib, a PARP inhibitor[14]. However, some research also suggests that EEC patients with PTEN defects respond more effectively to a combination therapy of PARP and PI3K inhibitors rather than single-agent PARP inhibitors[11].
03 PIK3CA
The protein encoded by PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha) is the catalytic subunit of PI3Ks, promoting cell proliferation by activating the PI3K/AKT/mTOR signaling pathway. PIK3CA gene mutations play a significant role in mediating tumorigenesis by enhancing the signal transduction of the PI3K/AKT/mTOR pathway and are associated with poor prognosis in various solid tumors[15]. The highest mutation frequency of the PIK3CA gene is observed in the POLE hypermutated subtype (71%), with frequencies around 50% in several other subtypes[1]. PIK3CA mutations and PIK3R1 mutations are usually mutually exclusive. In the NSMP subtype, patients with PTEN-PIK3CA co-mutations have significantly shorter overall survival (OS) than patients with PTEN-PIK3R1 co-mutations (P < 0.0005)[13].
Tumor patients with PIK3CA gene mutations are more sensitive to PI3K or mTOR inhibitors. The FDA has approved alpelisib in combination with fulvestrant for hormone receptor-positive (HR+), HER2-, PIK3CA-mutated advanced or metastatic breast cancer in postmenopausal women or men. FDA-approved PIK3CA genomic tissue or plasma testing is used as a companion diagnostic[16]. Several pan-cancer studies targeting this pathway are ongoing. For example, in Phase I study (NCT01219699) of alpelisib targeting advanced heavily pre-treated patients, the objective response rate (ORR) was only 6.0%, with one complete response (CR) observed in an EC patient and another EC patient achieving partial response (PR)[17]. A Phase II clinical study (NCT02549989) of Samotolisib (LY3023414) targeting advanced EC patients demonstrated an ORR of 16.0%, a clinical benefit rate (CBR) of 28%, with 4 patients achieving PR, 2 of them having responses lasting more than 9 months. The median progression-free survival (mPFS) and median overall survival (mOS) were 2.5 months and 9.2 months, respectively[18].
04 KRAS
Kirsten rat sarcoma viral oncogene homolog (KRAS) is one of the most common cancer genes in humans and encodes a small guanosine triphosphatase (GTPase). This protein cycles between an active GTP-bound state and an inactive guanosine diphosphate (GDP)-bound state, and it is associated with various downstream pathways, playing a significant role in cell proliferation, differentiation, and survival. The most typical pathways it's involved in include the MAPK and PI3K/AKT pathways[19].
▲KRAS Signaling Pathway and Inhibitors [19]
KRAS gene mutations mainly occur in the POLE and MSI-H subtypes, with frequencies of 53% and 35%, respectively. KRAS mutations are low-frequency in the NSMP subtype and are mutually exclusive with CTNNB1 gene mutations. They are rare in the CN-H subtype[1]. Studies suggest that KRAS mutations primarily occur in Type I (estrogen-dependent) EC, participating in early carcinogenesis, and are associated with increased cell proliferation, apoptosis, and upregulation of estrogen receptor (ER) in endometrial cells. The promoter of the KRAS gene can be influenced by high methylation, which may be associated with dMMR/MSI-H caused by MLH1 methylation. The role of KRAS in the transition from hyperplasia to EC and the invasive potential of Type I EC is well-established. Some researchers propose using KRAS as a prognostic stratification and early screening marker[20].
KRAS has been proven to be a challenging target for drug intervention. However, in recent years, several KRAS G12C inhibitors (including Sotorasib and Adagrasib) have demonstrated clinical benefits and have been approved by the FDA for non-small cell lung cancer (NSCLC)[16]. Some clinical studies are also ongoing. For example, the Sotorasib study targeting KRAS G12C mutations across various cancers (NCT03600883) has enrolled 2 EC patients with KRAS G12C mutations, with 1 achieving a partial response (PR) and a treatment duration of 6.9 months[21].
05 HER2
HER2 (ERBB2), erb-b2 receptor tyrosine kinase 2, is an EGFR receptor tyrosine kinase that activates the PI3K/AKT/mTOR and MAPK pathways, regulating growth and transformation[22]. About 30% of uterine serous carcinoma (USC) or 25% of CN-H subtype EC cases exhibit HER2 overexpression/amplification[1][23], suggesting that these patients may benefit from anti-HER2 therapies.
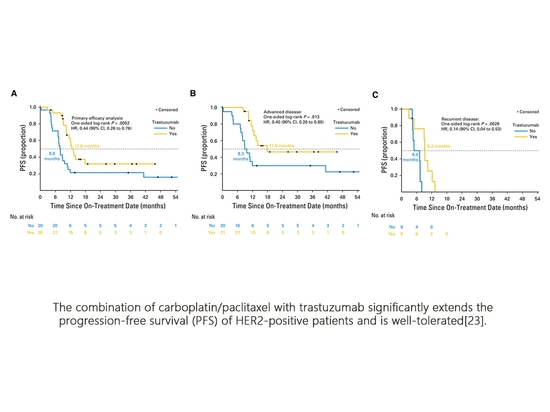
In a multicenter Phase II randomized study (NCT01367002), the efficacy of carboplatin/paclitaxel with or without trastuzumab was evaluated in HER2-positive advanced/recurrent patients, with the primary endpoint being progression-free survival (PFS). A total of 61 patients were randomly assigned, and efficacy was assessable in 58 patients. In the intention-to-treat population, the median PFS (mPFS) for the treatment group vs. the control group (Figure A) was 12.6 months vs. 8.0 months (HR=0.44, P=0.0052); for 41 Stage III/IV untreated patients (Figure B), mPFS was 17.9 months vs. 9.3 months (HR=0.40, P=0.013); and for 17 recurrent patients (Figure C), mPFS was 9.2 months vs. 6.0 months (HR=0.14, P=0.003). There were no differences in toxicity between the treatment and control groups, and no other safety signals were observed[23].
The combination of carboplatin/paclitaxel with trastuzumab significantly extends the progression-free survival (PFS) of HER2-positive patients and is well-tolerated[23].
NCCN guidelines recommend testing the HER2 status in TP53-mutated EC regardless of histological type. In terms of treatment, carboplatin + paclitaxel + trastuzumab is preferentially recommended for first-line systemic therapy in HER2-positive Stage III/IV USC (Category 2A evidence) or carcinosarcoma (Category 2B evidence) and for first-line therapy in recurrent HER2-positive USC (Category 2A evidence) or carcinosarcoma (Category 2B evidence)[10].
Furthermore, a Phase I multicenter study (NCT02564900) suggests that HER2-overexpressing or gene-mutated advanced solid tumor patients might consider treatment with trastuzumab deruxtecan. Two EC patients enrolled in the study both achieved partial responses (PR)[24].
06 MLH1
MLH1 is one of the four common mismatch repair (MMR) genes. Methylation of the MLH1 gene is a significant cause of deficient mismatch repair (dMMR). Approximately 8.2% of EC, 39.8% of dMMR EC, and 70.5% of EC with MLH1 and/or PMS2 deficiency are associated with MLH1 gene methylation[25]. The methylation status of the MLH1 gene promoter could serve as a marker for further stratification, with tumors experiencing MLH1 gene promoter methylation having worse prognosis compared to tumors with MMR gene mutations[25-26].
MLH1 methylation in EC leads to shorter overall survival (OS) and progression-free survival (PFS) compared to Lynch-related (Lynch-like) and pMMR EC[25].
In a single-arm, open-label Phase II study (NCT02899793), the efficacy of pembrolizumab was evaluated in recurrent dMMR/MSI-H endometrial cancer patients. The treatment involved intravenous injections of 200 mg every 3 weeks for 24 months. The primary endpoints were objective response rate (ORR) and toxicity based on RECIST v1.1 criteria, while secondary endpoints included PFS and OS.
The study enrolled 6 Lynch-like patients (25%) and 18 sporadic patients (75%). Among them, 19 patients exhibited MLH1 promoter methylation, 6 patients were dMMR based on immunohistochemistry (IHC), and 1 patient had both characteristics. After a median follow-up of 25.8 months, the ORR was 58% (95% CI, 36.6%-77.9%). The ORR was 100% for Lynch-like tumors and only 44% for sporadic tumors (p=0.024). The 3-year PFS was 100% compared to 30% (p=0.017), and OS was 100% compared to 43% (p=0.043). This study demonstrated that comparing the ORR, PFS, and OS of Lynch-like and sporadic MSI-H endometrial cancer, there are significant prognostic implications[27].
07 Summary:
In addition to the important auxiliary subtype genes discussed above, genes such as PIK3R1, FBXW7, and ARID1A are also frequently mutated in EC[1], although some of their roles are not yet fully understood. Gene mutations or fusions involving AKT1, FGFR2, NTRK, and others are associated with targeted therapies for advanced EC patients. Therefore, expert consensus recommends using NGS methods to test for more targets, seeking opportunities for cross-cancer indications, and participating in pan-cancer clinical trials[3].
In addition to offering endometrial cancer molecular subtyping, SpaceGen has also launched a new service for endometrial cancer genetic testing (53 genes + MSI), including subtyping, targeted therapy, chemotherapy, genetic-related genes, as well as immune therapy markers and positive/negative regulatory factors. This comprehensive service covers the common markers that EC patients need to be tested for. SpaceGen is dedicated to providing the most innovative products and services for personalized and precise medical testing in oncology, and continues to update its existing products.
Reference
[1] Nature. 2013 May 2;497(7447):67-73.
[2] Lancet Oncol. 2014 Jun;15(7):e268-78.
[3] Chinese Expert Consensus on Molecular Testing for Endometrial Cancer (2021 Edition)
[4] Front Oncol. 2021 Sep 1;11:612450.
[5] Mod Pathol. 2017 Jul;30(7):1032-1041.
[6] Clin Cancer Res. 2016 Aug 15;22(16):4215-24.
[7] Int J Gynecol Cancer. 2020 Dec;30(12): 2002-2007.
[8] Nat Rev Cancer. 2019 Sep; 19(9): 510-521.
[9] J Clin Oncol. 2015 Mar 10;33(8):930-6.
[10] NCCN Uterine Neoplasms Guidelines 2023 Version 2
[11] Oncogene. 2018 Jan 18;37(3):341-351.
[12] Trends Biochem Sci. 2014 Apr;39(4):183-90.
[13] Mod Pathol. 2022 Sep;35(9):1269-1278.
[14] Nat Rev Clin Oncol. 2011 May;8(5):302-6.
[15]Cancers (Basel). 2019 Dec 30;12(1):93.
[16] FDA Official Database
[17] J Clin Oncol. 2018 May 1;36(13):1291-1299.
[18] Cancer. 2020 Mar 15;126(6):1274-1282.
[19] Cancers (Basel). 2021 Jan 5;13(1):151.
[20] Anticancer Res. 2019 Feb;39(2):533-539.
[21] N Engl J Med. 2020 Sep 24;383(13):1207-1217.
[22] Oncogene. 2007 Oct 4;26(45):6469-87.
[23] J Clin Oncol. 2018 Jul 10;36(20):2044-2051.
[24] Cancer Discov. 2020 May;10(5):688-701.
[25] J Gynecol Oncol. 2021 Nov;32(6):e79.
[26] Mod Pathol. 2020 Jul;33(7):1443-1452.
[27] Ann Oncol. 2021 Aug;32(8):1045-1046.
Disclaimer: This article is for sharing purposes only and does not represent the platform's stance. If there are any copyright or other issues, please contact us promptly, and we will make corrections as soon as possible. Thank you!