#Product Trends
The relationship between thyroid cancer and BRAF gene
The relationship between thyroid cancer and BRAF gene
In recent years, the incidence of thyroid cancer has continued to rise rapidly in many countries and regions around the world. There are nearly 600,000 new cases worldwide each year, and Chinese patients account for more than one-third of them (Figure 1). The ratio of female to male patients is about 3:1 [1].
Figure 1 The top ten cancers with the most new cancer cases in China in 2020
Thyroid cancer is the most common malignant endocrine tumor and originates from thyroid follicular epithelial or parafollicular epithelial cells (Figure 2). Based on tumor origin and differentiation, thyroid cancer can be divided into differentiated thyroid cancer (DTC), medullary thyroid cancer (MTC), poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC). DTC is further divided into papillary thyroid cancer. thyroid cancer (PTC) and follicular thyroid cancer (FTC) [2]. Among all tissue types, PTC is the most common, accounting for approximately 90% of all thyroid cancers. Studies have shown that the most common mutated gene in PTC is BRAF, with a mutation rate of up to 80%; while mutations are less common in ATC, and mutations are rare in benign nodules, FTC, MTC, and PDTC [3]. Since the initial discovery of BRAF gene mutations in human cancers, more than 40 mutation sites have been identified, among which the T1799A site is the most common BRAF mutation, accounting for more than 90% of all BRAF gene mutations. The T1799A site is located on exon 15 of the BRAF gene. The mutation at this site causes the valine (V) at position 600 in the protein product to be replaced by glutamic acid (E) (named V600E), thereby activating the BRAF kinase. causing carcinogenesis [4]. BRAF V600E mutation is highly related to PTC and is a very important tumor marker for PTC. The risk of malignant thyroid nodules with BRAF mutation reaches 99.8% [3]. At the same time, BRAF mutations are closely related to the clinical progression, recurrence, and iodine resistance of PTC.
Figure 2 Thyroid
BRAF mutation and clinical progression of PTC
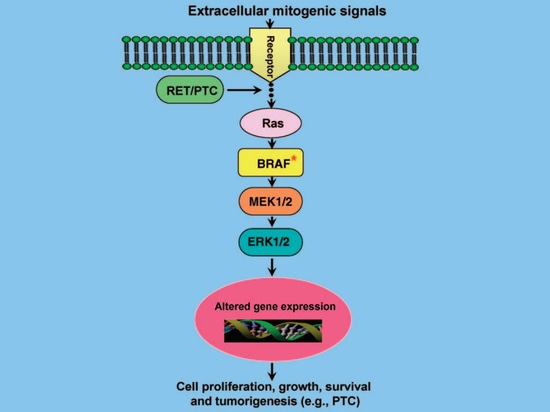
The BRAF gene encodes a serine/threonine protein kinase and is an important component of the MAPK signal transduction pathway (RAS-RAF-MEK-ERK/MAPK kinase pathway). This signaling pathway plays an important role in cell proliferation, differentiation, apoptosis and other cell functions. When abnormally activated, tumors occur. In normal cells, the physiological activation of this pathway is triggered by a large number of growth factors, hormones and cytokines through their receptors on the cell membrane, and then activates RAF kinase through GTP-RAS, thereby activating downstream MEK, and then activating downstream ERK, activated ERK phosphorylates regulatory protein molecules in the nucleus, and ultimately changes gene expression, thereby changing the biological activity of the cell (Figure 3) [5]. Therefore, when the BRAF gene is mutated, it will continue to remain active, continuously activating the MAPK pathway in tumor cells, and the growth and development of cells will lose control, eventually leading to tumor occurrence [5]. In addition, studies have shown that BRAF mutations induce abnormal methylation of PTC-related tumor suppressor genes (TIMP3, DAPK, SLC5A8, RARβ2, etc.), accelerating tumor progression [6]. For example, the TIMP3 tumor suppressor gene can inhibit tumor growth, angiogenesis, invasion and metastasis, and block the binding of vascular endothelial growth factor (VEGF) to the VEGF receptor; however, BRAF mutations mediate methylation of the TIMP3 gene, leading to gene silencing. This results in the loss of gene function and promotes the progression and invasiveness of PTC [7].
Figure 3 MAPK signaling pathway
BRAF mutation and PTC recurrence
Most studies have shown that BRAF mutations increase the risk of PTC disease recurrence regardless of risk levels associated with clinicopathological, geographic, and ethnic factors. Xing et al. conducted a multi-center study on 219 PTC patients and retrospectively analyzed the relationship between BRAF mutations and tumor recurrence in primary PTC. The results confirmed that BRAF mutations are closely related to PTC recurrence [8]. A study by Kim et al also showed that in 203 patients with primary PTC, BRAF mutation was closely related to tumor recurrence [9].
Figure 4 Comparison of recurrence-free probabilities in PTC patients with BRAF gene mutations and those without mutations
BRAF mutation and PTC iodine resistance
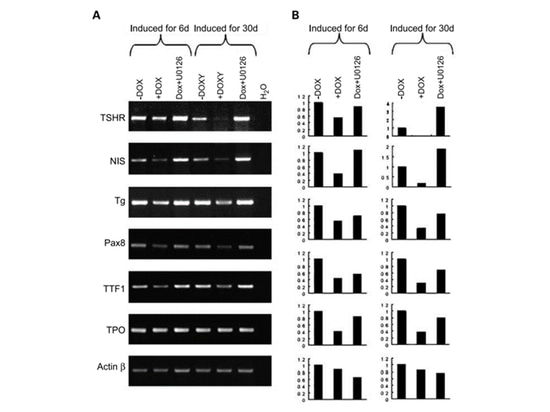
After thyroidectomy, radioactive iodine ablation is the primary treatment for PTC. This radioactive iodine therapy utilizes the sodium/iodide cotransporter (NIS) of thyroid cells to absorb, concentrate, and organize iodide. This process requires the participation of thyrotropin receptor (TSHR), thyroglobulin (Tg), thyroid peroxidase (TPO), and the transcription factors TTF-1 and Pax-8 [4]. However, the expression of these genes related to iodine metabolism is often damaged or lost in primary or recurrent PTC, and studies have found that this is related to BRAF mutations [10]. One study [10] induced the expression of BRAF V600E and changed the MAPK pathway activity, and then detected the expression of thyroid iodine metabolism genes. By inducing the expression of BRAF V600E with DOX (doxycycline) for 6 days, the expression of TSHR, NIS, Tg and PAX8 genes related to iodine metabolism were all down-regulated; and by adding U0126 (MEK inhibitor), the expression of the down-regulated genes could be restored. After long-term induction of BRAF V600E (30 days), these silenced thyroid iodine metabolism genes could still be restored by U0126 treatment. The research results show that targeting the RAF-MEK-MAP pathway therapy is a good way to restore the iodine metabolism ability of PTC carrying BRAF mutations.
Figure 5 Study on the expression of iodine metabolism genes
In summary, BRAF gene mutations are closely related to papillary thyroid cancer (PTC), can be used as a predictive marker for PTC prognosis, and are also closely related to patient prognosis. BRAF gene testing has obvious clinical value in PTC. In addition to its prognostic value, it can also evaluate the malignant risk of thyroid nodules, guide the selection of surgical plans, and guide targeted therapy. However, there may also be RET/PTC rearrangements, Ras mutations, etc. in the MAPK pathway, and they are mutually exclusive with BRAF gene mutations [11].
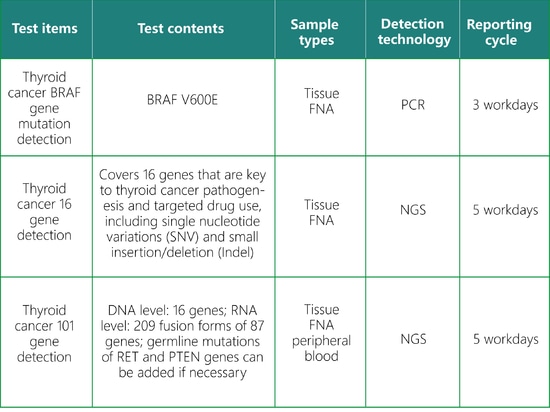
SpaceGen Thyroid Cancer Gene Testing
SpaceGen's thyroid cancer gene mutation detection is conducive to scientifically and accurately assessing an individual's risk of thyroid cancer and formulating a personalized management plan.
References
[1] IARC 2020 latest global cancer burden data.
[2] Thyroid Cancer Diagnosis and Treatment Guidelines (2022 Edition)
[3] Guangdong Expert Consensus on Gene Testing and Clinical Application of Thyroid Cancer (2020 Edition)
[4] Critical Reviews in Oncology/Hematology, 2014, 90(3).
[5] Endocrine Reviews 28(7):742–762.
[6] J Clin Endocrinol Metab 90:3028 –3035.
[7] Biochem Cell Biol 74:853– 862.
[8] J Clin Endocrinol Metab 90:6373– 6379.
[9] Clin Endocrinol (Oxf) 65:364 –368.
[10] Clin Cancer Res, 2007, 13(4): 1341-1349.
[11] CSCO Diagnosis and Treatment Guidelines for Differentiated Thyroid Cancer (2021 Edition)
Statement: This article is for sharing only and does not represent the position of the platform. If there are copyright and other issues involved, please contact us as soon as possible and we will correct it as soon as possible. Thank you!