#Industry News
SpaceGen’s sequencing service supports research on the treatment of adult gastric isolated Langerhans cell histiocytosis
SpaceGen’s sequencing service supports research on the treatment of adult gastric isolated Langerhans cell histiocytosis
Cutting-edge academic information, scientific research results express
Recently, the Chinese Journal of Pathology published a short article "Isolated Langerhans cell histiocytosis of the stomach in adults: a clinicopathological analysis of three cases", which attracted widespread attention. This article mainly discusses the clinical manifestations, pathological characteristics, immunophenotype, molecular pathology, differential diagnosis and treatment prognosis of isolated Langerhans cell histiocytosis (LCH) in adults. SpaceGen provided high-throughput sequencing technology for this study to detect and analyze the hot mutation regions of BRAF, KRAS and NRAS genes. It is once again confirmed that SpaceGen has leading and reliable quality control and testing capabilities, which can provide a strong guarantee for precise clinical diagnosis and treatment.
This article is quoted below
Isolated Langerhans cell histiocytosis of the stomach in adults: a clinicopathological analysis of three cases
Chen Xi1 Yuan Jingping1 Zhao Lina1 He Huihua1 Ao Qilin2 Ju Xianli1 Chen Fangfang1
1 Department of Pathology, Renmin Hospital of Wuhan University, Wuhan 430060; 2 Pathology, Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology
Research Institute, Wuhan 430030
Corresponding author: Chen Fangfang
Summary
Objective: To explore the clinical manifestations, pathological characteristics, immunophenotype, molecular pathology, differential diagnosis and treatment prognosis of isolated Langerhans cell histiocytosis (LCH) in adults.
Methods: The clinicopathological characteristics, immunophenotype and molecular pathology of 3 cases of gastric solitary LCH were analyzed, and relevant literature was summarized.
Results: Case 1, a 23-year-old male, had a polyp-like bulge on the anterior wall at the junction of the gastric antrum and body during gastroscopy; Case 2, a 22-year-old male, had a bulge and erosion on the lesser curvature of the gastric body; Case 3, a 46-year-old male, had a gastric bulge found on the lesser curvature of the gastric body. There was a hyperemia and erosion focus in the upper part of the stomach body and a polyp in the middle part. Abnormally proliferated Langerhans cells were seen under the microscope in all 3 cases. The cells were of medium size, with slightly eosinophilic cytoplasm, oval nuclei, and nuclear grooves could be seen. There were a large number of inflammatory cell infiltrates in the background. Immunohistochemistry showed CD1α, Langerin, S‐100 protein was all positive. Cases 2 and 3 detected BRAF V600E gene mutation. All 3 cases underwent chest and abdominal CT or positron emission computed tomography (PET‐CT) to rule out LCH of other systems.
Conclusion: Gastric solitary LCH is very rare and has a good prognosis. Inexperienced doctors often do not fully understand the key points of diagnosis of this disease. They should pay attention to distinguishing it from poorly differentiated adenocarcinoma to avoid misdiagnosis as a malignant tumor.
Fund project: Wuhan Science and Technology Planning Project (2017060201010172); Wuhan University People’s Hospital Guidance Fund (RMYD2018M27)
Langerhans cell histiocytosis (LCH) is a rare disease characterized by abnormal proliferation of Langerhans cells [1]. It mostly occurs in children and often involves multiple systems [2]. Isolated gastric LCH in adults is rare and poorly studied. This study collected 3 cases of adult gastric solitary LCH, reviewed relevant literature, and explored the clinicopathological characteristics, immunophenotype, molecular pathology, and differential diagnosis of adult gastric solitary LCH, aiming to improve clinical and pathologist understanding of this disease. know.
1. Materials and methods
1. Case data: The clinical pathological data of 3 patients diagnosed with adult gastric isolated LCH from December 2017 to December 2022 were collected and analyzed from the Department of Pathology of Wuhan University People's Hospital and the Institute of Pathology of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology. Its histological morphology, immunophenotype, molecular pathology, and follow-up.
2. Methods: The specimens were fixed with 3.7% neutral formaldehyde, dehydrated routinely, embedded in paraffin, sectioned into 4 μm thick sections, stained with HE, and observed under a light microscope. Immunohistochemistry used the EnVision method, and the antibodies used were purchased from Dako, including CD1α, Langerin, S-100 protein, cyclin D1, Ki-67, broad-spectrum cytokeratin (CKpan), CD56, and chromogranin A (CgA). , synaptophysin, p53, CD20, CD79α, CD3, CD30, HMB45, Melan A, SOX10, all ready-to-use antibodies. High-throughput sequencing technology was used to detect the hot mutation regions of BRAF, KRAS and NRAS genes. The detection platform was the DA8600 second-generation sequencer, and sequencing services were provided by Xiamen SpaceGen Co., Ltd.
2. Results
1) Clinical data: Case 1, a 23-year-old male, was admitted to the hospital for treatment due to "chest pain and 18 days after closed chest drainage". Gastroscopy showed that polypoid bulges were visible on the anterior wall of the junction of the gastric antrum and body, with a size of about 0.3 cm × 0.3 cm. The surface is smooth, the boundaries are clear, the texture is soft, and no obvious abnormalities are found. Case 2, a 22-year-old male, was admitted to the hospital for treatment due to "hunger pain for 3 days". Gastroscopy showed that a raised erosion was seen on the lesser curvature of the stomach, which was soft in texture, but no other obvious abnormalities were found. Case 3, a 46-year-old male, was admitted to the hospital due to "intermittent epigastric distension for 2 months". Gastroscopy showed that there was a congestive and erosive focus in the upper part of the gastric body near the cardia, and a 0.3 cm × 0.3 cm polyp was found on the greater curvature side of the middle part. The rest was not obvious. abnormal.
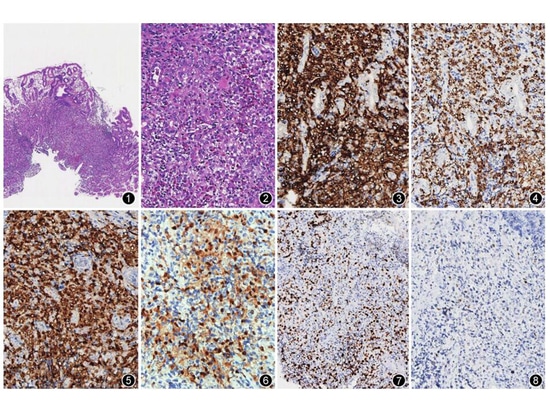
2) Pathological examination: (1) Gross examination: Case 1, a piece of gray-white tissue, 0.2 cm in diameter. Example 2, a piece of gray-white tissue with a diameter of 0.3 cm. Example 3, 2 pieces of gray-white tissue, 0.2~0.3 cm in diameter. (2) Histological characteristics: In all 3 cases, abnormal proliferation of Langerhans cells was found in the lamina propria of the gastric mucosa (Figure 1). The cells were medium in size, with slightly eosinophilic cytoplasm, oval nuclei, unclear nucleoli, and visible nuclear grooves; inflammatory cells such as eosinophils, lymphocytes, and plasma cells were seen in the background (Figure 2). (3) Immunohistochemistry: 3 cases were positive for CD1α (Figure 3), Langerin (Figure 4), and S-100 protein (Figure 5), the Ki-67 positive index was approximately 20% (Figure 6), and CKpan was negative. Example 1, CgA, CD56, synaptophysin, CD20, CD79α, CD3, CD30, HMB45, Melan A, and SOX10 were all negative; Example 2, cyclinD1 (Figure 7) was positive, synaptophysin, CD56, and CgA were negative, and p53 individual cells Positive (Figure 8); Example 3, cyclin D1 positive, p53 individual cells positive. (4) Gene testing: The BRAF V600E gene mutation was detected in the primary lesions of Cases 2 and 3, but the KRAS and NRAS genes were not mutated.
Figure 1 Gastric solitary Langerhans cell histiocytosis. Tumor cells are surrounded by dense glandular HE of the gastric mucosa lamina propria. Low magnification. Figure 2 Tumor cells show typical coffee bean-like nuclei and a large number of inflammatory cells in the background. Infiltrating HE medium magnification Figure 3 Langerhans cells CD1α positive EnVision medium medium magnification Figure 4 Langerhans cells Langerin positive EnVision medium magnification Figure 5 Langerhans cells S-100 protein positive EnVision medium magnification Figure 6 The Ki-67 positive index of Langerhans cells is about 20%. Medium magnification by EnVision method. Figure 7 Langerhans cells are positive for cyclin D1. High magnification by EnVision method. Figure 8 Individual cells of Langerhans cells are positive for p53. Medium magnification by EnVision method.
3) Other examinations: No obvious abnormalities were found in the postoperative chest and abdominal CT scans of Cases 1 and 3. In Case 2, no tumor cells were found in the bone marrow examination and no gene mutation was detected in the bone marrow. A whole-body positron emission computed tomography (PET-CT) scan showed no obvious abnormalities. In all 3 cases, involvement of other systems was excluded and they were isolated.
4) Treatment and follow-up: Patients in Case 1 and Case 3 underwent conservative treatment after biopsy, and are in good condition so far during follow-up. Patient 2 underwent biopsy and underwent gastroscopy again and underwent endoscopic submucosal dissection (ESD). During the operation, an erosion of 1.2 cm × 0.8 cm was found in the lesser curvature of the gastric body. No tumor cells were found in all post-operative tissue samples. All patients were in good condition during follow-up for 6 months to 5 years.
3. Discussion
In the past, LCH was considered to be three separate diseases: eosinophilic granulomatosis of bone, Letterer‐Siwe disease, and Schüller‐Christian disease. Because the three have many similarities in clinical pathological characteristics, Lichtenstein [3] in 1953 collectively referred to them as histiocytosis X. In 1973, some scholars discovered that histiocytosis LCH[4-5].
The range of involvement of LCH lesions is variable, and it is clinically divided into three categories: single focus and single system, multifocal single system, and multifocal and multisystem [6]. Among them, adult LCH that occurs solely in the stomach is extremely rare. We found it in Wanfang and PubMed databases. 15 cases were found [6-13], the patients were aged 28 to 68 years old, and the male to female ratio was 8:7. Eight of the patients were asymptomatic, and seven patients had gastrointestinal symptoms including stomach discomfort (3 cases), upper abdominal pain (2 cases), dysphagia (1 case), and nausea/vomiting (1 case). Solitary bulges or polyps (size 0.2~1.0 cm) were seen in 10 cases under gastroscopy, and 1 case had multiple polyps. Other lesions included erosion (2 cases) and ulcers (1 case). Only 1 case had no lesions. The most common site was the gastric body (7 cases), and other sites included the gastric antrum (4 cases), gastric fundus (1 case), and gastric angle (1 case). There was also 1 case with lesions in both the gastric body and the gastric fundus, and 1 case with lesions. The lesions were diffusely distributed throughout the stomach. In this group of cases, Case 1 had no corresponding gastrointestinal symptoms, Case 2 showed hunger pain, and Case 3 showed upper abdominal distension. All 3 cases were male, aged 22, 23, and 46 years old respectively. Under gastroscopy, they were all located in the body of the stomach. of solitary polyps or bumps. The clinical characteristics of the three cases were similar to previously reported cases, and the age of onset was younger, broadening the age range of its onset.
Some studies have proposed that the key points for diagnosing LCH are: having specific microscopic manifestations and related clinical/imaging manifestations, and performing at least two immunohistochemical tests of CD1α and Langerin [14]. Histologically, LCH is characterized by abnormally proliferated Langerhans cells nested in a sheet-like distribution. The cells are medium in size, with round or oval nuclei, indistinct nucleoli, nuclear membrane folds, and coffee bean-like nuclear grooves. The quality is slightly eosinophilic[15]. There is often infiltration of reactive inflammatory cells such as eosinophils, lymphocytes, and plasma cells in the background. On immunohistochemistry, tumor cells specifically expressed CD1α, S‐100 protein, and Langerin. Another study on cutaneous LCH found that 92.3% to 100% of cases had strong expression of cyclin D1 in tumor cell nuclei, with a positive rate of 5% to 70%, while cyclin D1 was negatively expressed in reactive lesions [16-18]. This suggests that cyclin D1 can be used to differentiate between LCH and reactive lesions. There is currently no study report on the positive rate of cyclin D1 in isolated gastric LCH, and there is no unified standard for the positive interpretation of cyclin D1. In this group, the cyclin D1 positive rates of Case 2 and Case 3 were 10% and 30% respectively. In addition, recent studies suggest whole-body fluorodeoxyglucose PET-CT in patients with LCH, including the distal extremities, to help determine the extent and extent of the disease. The microscopic characteristics, immunohistochemistry and imaging results of the 3 cases in this group all met the above requirements.
Possible molecular characteristics of adult LCH include: (1) BRAF V600E mutation; (2) other activating mutations in the RAS-RAF-MEK-ERK pathway; (3) activating kinase fusion [14]. Among them, the BRAF V600E mutation is present in more than half of LCH cases [18]. Of the 15 cases retrieved, only 6 were tested for BRAF V600E mutations, and mutations were present in 5 of them. In this group, Cases 2 and 3 used high-throughput sequencing technology to detect mutations in the BRAF V600E gene, but no mutations in the KRAS and NRAS genes. Combining previous cases and 2 cases in this group, the proportion of BRAF V600E mutations in adult gastric isolated LCH is 7/8, and more data need to be further studied.
The differential diagnosis of this disease is: (1) Erdheim-Chester disease: microscopic examination shows mild xanthogranulomatous inflammation and varying degrees of fibrosis, often with strong expression of factor XIIIa and CD163, but no expression of S-100 protein; (2) Rosai‐Dofman disease: tumor cells lack nuclear grooves and express S‐100 protein and CD163; (3) poorly differentiated cancer: tumor cells have large atypia, frequent mitoses, and express CKpan; (4) Lymphoma: tumor cells express corresponding Markers of B cells and T cells, but do not express S-100 protein. None of the above four diseases express CD1α and Langerin. It should be noted that the Ki-67 positive index of LCH varies greatly. The positive index of the three cases in this group was about 20%, while it could reach up to 60% in previously reported cases [8]. It is important to avoid misdiagnosis as a malignant tumor.
The prognosis of LCH is closely related to the number of organs involved, the number of dangerous organs involved, and the onset [19]. Patients with single-focus and single-system LCH generally only require local resection or observation. In the past, 15 cases of isolated gastric LCH underwent surgical resection (2 cases), ESD (4 cases) or biopsy (9 cases). The follow-up time ranged from 3 months to 5.5 years, and the condition was good during the follow-up period. In this group, patients 1 and 3 were observed after biopsy, and patient 2 underwent biopsy and ESD treatment. All three patients were followed up for 6 months to 5 years and were in good condition, which is consistent with the treatment methods and prognosis reported in the past.
It can be seen from the above that solitary LCH in the stomach in adults is rare. Most patients are asymptomatic or have mild gastrointestinal symptoms. Most patients show solitary polyps or bulges under gastroscopy. Microscopic features, immunohistochemistry, whole-body PET-CT to exclude other system involvement, and molecular pathology suggest that BRAF V600E gene mutation is a necessary means to confirm the diagnosis of this disease. Treatment is generally local excision or biopsy, and the overall prognosis is good. Due to the microscopic characteristics and large changes in Ki-67 positive index, misdiagnosis as malignant tumors such as poorly differentiated carcinoma should be avoided.
References:
[1] Andión Catalán M, Ruano Domínguez D, Azorí Cuadrillero D, et al. Gastrointestinal involvement in Langerhans cell histiocytosis[J].An Pediatr (Barc), 2015,83(4):279280. DOI: 10.1016/j.anpedi.2015.04.014.
[2] Yoon HS, Lee JH, Michlitsch J, et al. Langerhans cell histiocytosis of the gastrointestinal tract: evidence for risk organ status[J]. J Pediatr, 2019, 212:6672.e3.DOI:10.1016/j.jpeds.2019.05.003.
[3] Lichtenstein L. Histiocytosis X; integration of eosinophilic granuloma of bone, LettererSiwe disease, and SchüllerChristian disease as related manifestations of a single nosologic entity[J]. AMA Arch Pathol, 1953, 56(1):84102.
[4] Lamert F. Langerhans cell histiocytosis. Historical perspectives[J]. Hematol Oncol Clin North Am, 1998,12(2):213219. DOI: 10.1016/s08898588(05)705062.
[5] Berry DH, Becton DL. Natural history of histiocytosisX[J].Hematol Oncol Clin North Am, 1987, 1(1):2334.
[6] Chen Hongmei, Ye Lin, Zhu Liangjun, et al. A case of isolated gastric Langerhans cell histiocytosis [J]. Journal of Clinical and Experimental Pathology, 2018, 34(9):1028 1029. DOI: 10.13315/ j.cnki.cjcep.2018.09.021.
[7] Wang L, Yang F, Ding Y, et al. Gastrointestinal Langerhans cell histiocytosis with unifocal, singlesystem involvement in adults: cases report and literature review[J]. J Clin Lab Anal, 2022, 36(12):e24765. DOI: 10.1002/jcla.24765.
[8]Zhou Xiaoli, Fan Li, Gu Wenxian, et al. A case of gastric Langerhans cell histiocytosis [J]. Chinese Journal of Pathology, 2022, 51(10): 1062 1064.DOI: 10.3760/cma.j.cn112151 20220319 00201.
[9] Wu Lihua, Li Chenfan, Zheng Yueping. A case of isolated gastric Langerhans cell histiocytosis [J]. Chinese Clinical Case Results Database, 2022, 04(01).
[10] Matsuoka Y, Iemura Y, Fujimoto M, et al. Upper 55Chinese Journal of Pathol, September 2023, Vol. 52, No. 9 Chin J Pathol, September 2023, Vol. 52, No. 9Gastrointestinal Langerhans cell histiocytosis: a report of 2 adult cases and a literature review[J]. Int J Surg Pathol, 2021, 29(5):550 556. DOI: 10.1177/1066896920964566.
[11] Li Yanan, Shao Shihong, Zhao Han, et al. A case of isolated gastric Langerhans cell histiocytosis [J]. Chinese Journal of Pathology, 2020, 49(6): 631 633.DOI: 10.3760/cma.j. cn112151 20191008 00545.
[12] Yan F, Zhou Q, Gao Y, et al. Isolated Langerhans cell histiocytosis of the stomach: a case report and literature review[J]. Int J Clin Exp Pathol, 2018, 11(12):59625968.
[13] Lee SJ, Hwang CS, Huh GY, et al. Gastric Langerhans cell histiocytosis: case report and review of the literature[J]. JPathol Transl Med, 2015, 49(5):421423. DOI: 10.4132/jptm.2015.05.19.
[14] Goyal G, Tazi A, Go RS, et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults[J].Blood, 2022, 139(17): 26012621. DOI: 10.1182/blood.2021014343.
[15] Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophagedendritic cell lineages[J]. Blood, 2016, 127(22): 26722681. DOI:10.1182/blood201601690636.
[16] Shanmugam V, Craig JW, Hornick JL, et al. Cyclin D1 is expressed in neoplastic cells of Langerhans histiocytosis but not reactive Langerhans cell proliferations[J]. Am J Surg Pathol, 2017, 41(10):13901396. DOI: 10.1097/PAS.0000000000000897.
[17] Chatterjee D, Vishwajeet V, Saikia UN, et al. CyclinD1 is useful to differentiate Langerhans cell histiocytosis from reactive Langerhans cells[J]. Am J Dermatopathol, 2019,41(3):188192. DOI: 10.1097/DAD.0000000000001250.
[18] Ben Rejeb S, Charfi L, Sahraoui G, et al. Cyclin D1:potential utility as marker for Langerhans cell histiocytosis[J]. J Immunoassay Immunochem, 2021,42(4):370379. DOI: 10.1080/15321819.2020.1870132.
[19] Selway JL, Harikumar PE, Chu A, et al. Genetic homogeneity of adult Langerhans cell histiocytosis lesions: Insights from BRAFV600E mutations in adult populations[J]. Oncol Lett, 2017, 14(4):44494454. DOI:10.3892/ol.2017.6774.
[20] Matsubara Y, Kobayashi M, Hijikata Y, et al.Gastrointestinal lesion in adultonset Langerhans cell histiocytosis[J]. Int J Clin Oncol, 2020, 25(11):19451950.