#Industry News
The 2023 FIGO staging of endometrial cancer includes molecular classification
The 2023 FIGO staging of endometrial cancer includes molecular classification
Since the release of the International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging system in 2009, a large amount of new literature has emerged that better defines the pathological and molecular findings associated with endometrial cancer types. In addition, there have been numerous reports on new treatments, clinical trial results, prognostic and survival data related to pathological and surgical outcomes.
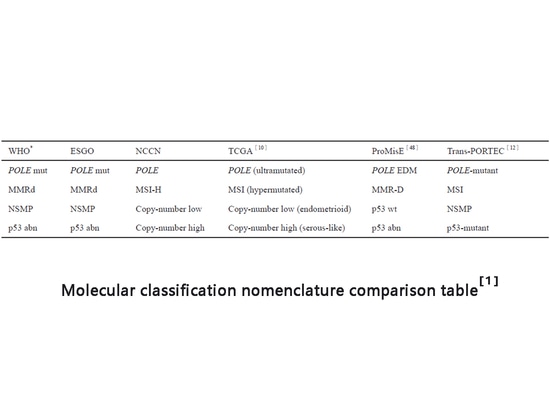
A major advance in the diagnosis and treatment of endometrial cancer over the past decade has been the development of molecular classification systems. Molecular characterization helps understand recurrence risk and survival outcomes. Among these systems, TCGA (The Cancer Genome Atlas) provides a comprehensive classification. Endometrial cancer can be divided into four different molecular categories using molecular detection methods: POLE hypermutated (POLEmut), Microsatellite instability or mismatch repair deficiency (MSI-H or MMRd), low copy or no specific molecular profile (CNL or NSMP), and high copy or p53 abnormality (CNH or p53abn). Each category corresponds to a different prognosis, with POLEmut generally having the best prognosis, p53abn having the worst prognosis, and the remaining two categories having an intermediate prognosis. Molecular classification is included in clinical diagnosis and treatment guidelines (NCCN, CSCO, etc.). Molecular classification detection methods include NGS (full typing), immunohistochemistry (MMR protein, p53 protein), and Sanger sequencing (POLE hotspot mutations). and fragment analysis (MSI), etc., no matter which detection or naming method is used, the four subtypes basically have a one-to-one correspondence.
Molecular classification nomenclature comparison table[1]
The integration of this molecular classification into the FIGO Endometrial Cancer Staging System in 2023 marks an important step in improving the ability to stratify endometrial cancer risk beyond the limitations of traditional histological assessment. This new development helps provide guidance for clinicians to determine patient treatment options.
The FIGO staging system updated in 2009 is widely used. However, as the understanding of endometrial cancer continues to deepen, it has been discovered that the 2009 FIGO staging system still has many shortcomings, including insufficient attention to the histological types of endometrial cancer. Neglect of lymphovascular space invasion (LVSI), lack of differentiation based on size of lymph node metastasis, unclear stage assignment for patients with endometrioid carcinoma involving both the endometrium and ovary, and missing molecular classification. The updated FIGO 2023 staging system based on the above shortcomings has corrected these limitations, further improved the staging of endometrial cancer, and further optimized the management of patients.
The 2023 FIGO staging of endometrial cancer[2]
The 2023 staging of endometrial cancer includes molecular classification
The 2023 FIGO staging of endometrial cancer indicates that when feasible, adding molecular subtypes to the staging criteria can better predict stage and prognosis. Complete molecular classification (POLEmut, MMRd, NSMP, p53abn) is encouraged in all endometrial cancer cases for prognostic risk group stratification and as a potential influencing factor in decisions about adjuvant or systemic therapy. Molecular subtyping can be done on biopsy, in which case it does not need to be repeated on hysterectomy specimens.
POLEmut indicates good prognosis, while p53abn indicates poor prognosis. In early-stage endometrial cancer, where pathogenic POLE mutations or p53 abnormalities affect FIGO staging, MMRd and NSMP indicate intermediate prognosis and therefore do not alter staging.
When the molecular classification is clarified, for FIGO stages I and II, it is initially based on surgical/anatomical and histological results and then should be revised by incorporating the molecular classification and adding a subscript (m represents molecular classification) to indicate this change, as shown in the figure below; for FIGO stage III, it is based on surgical/anatomical results, and the stage category does not need to be corrected by molecular profiling. However, when molecular classification indicates p53 abnormality, in the purpose of data collection, it should be recorded as stage IIImp53abn; for FIGO stage IV, based on surgical/anatomical results, there is no need to correct the staging category through molecular characterization analysis, but it also needs to be recorded for data collection.
The 2023 staging of endometrial cancer includes molecular classification[2]
Summary
As molecular classification is incorporated into the FIGO 2023 staging system, understanding of endometrial cancer continues to increase, and the important role of histological subtyping, LVSI, lymph node metastasis size, and the need to differentiate between synchronous and metastatic cancers in utero indicate the complexities of staging pancreatic cancer. The new version of FIGO staging is more detailed for the staging of endometrial cancer, more organized and logical, more practical, close to clinical reality, and in line with the essence of precision medicine. In the future, more research will be conducted to further improve the staging management of endometrial cancer patients, in order to better stratify, guide subsequent treatment, and ultimately bring survival benefits to patients.
SpaceGen TypesenTM Endometrial Cancer Molecular Classification detection
SpaceGen TypesenTM human endometrial cancer molecular classification detection service is based on SpaceGen's patented RingCap® technology, which can provide independent prognostic information for endometrial cancer and provide a reference for adjuvant treatment and conservation therapy. It includes MMR gene mutation and MSI status detection, which can indicate the efficacy of immune checkpoint inhibitors and screen for Lynch syndrome.
Detection content
This detection uses a high-throughput sequencing method to cover 12 genes related to molecular typing of endometrial cancer, including POLE, TP53, MLH1, MSH2, PMS2, and MSH6, as well as MSI-related sites.
Detection advantages
1. Scientific rigor: Molecular classification is included in NCCN guidelines;
2. Simple and fast: With independent patented RingCap® technology, library construction can be completed in just two steps;
3. High sensitivity: 5000X sequencing depth, sensitivity as high as 1%;
4. Comprehensive detection: 12 genes related to molecular typing of endometrial cancer and 34 MSI sites are detected at one time, with wide coverage.
References
[1]Chinese expert consensus on molecular detection of endometrial cancer (2021 edition)
[2][2]Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D, Concin N; Endometrial Cancer Staging Subcommittee, FIGO Women's Cancer Committee. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023 Aug;162(2):383-394. doi: 10.1002/ijgo.14923. Epub 2023 Jun 20. PMID: 37337978.
Statement: This article is for sharing only and does not represent the position of the platform. If there are copyright and other issues involved, please contact us as soon as possible and we will correct it as soon as possible. Thank you!