#Industry News
Summary of updates on ovarian cancer indications for PARP inhibitors
Summary of updates on ovarian cancer indications for PARP inhibitors
Preface
Polyadenosine diphosphate ribose polymerase (PARP) plays a key role in the process of DNA single-strand base excision and repair. In homologous recombination repair deficient (HRD) tumor cells, the double-stranded DNA cannot be repaired, and PARP inhibitors block single-strand repair, resulting in a "synthetic lethal" effect, leading to tumor cell death [1]. Some indications of PARP inhibitors require biomarkers such as BRCA1/2 gene mutations or HRD status as companion diagnostics, and some indications are effective for the entire population. The indications for PARP inhibitors have been changing over the past few years, and this article attempts to summarize some of the updates.
First-line maintenance therapy for ovarian cancer
The U.S. Food and Drug Administration (FDA) approved olaparib monotherapy, niraparib monotherapy, and the combination of olaparib with bevacizumab for first-line maintenance therapy indications on December 19, 2018, April 29, 2020, and May 8, 2020, respectively. Olaparib monotherapy's biomarker for maintenance therapy is the presence of BRCA1/2 gene germline or somatic mutations (g/sBRCAm), and olaparib in combination with bevacizumab is indicated for HRD[2]. Based on data from the PRIMA study (NCT02655016), niraparib demonstrated benefit in the overall population without the requirement for concurrent diagnostics during maintenance therapy. However, the benefit in HRD population was superior to homologous recombination proficient (HRP) population, particularly among those with BRCA mutations[3]. The National Medical Products Administration (NMPA) in China also approved these three first-line maintenance therapy indications, with olaparib monotherapy and niraparib monotherapy being included in medical insurance coverage[4].
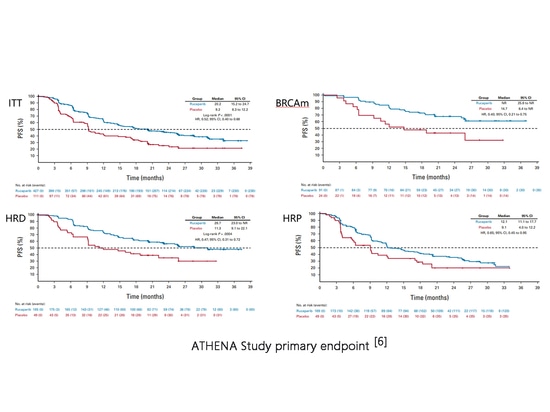
In the first edition of the 2023 National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer, fallopian tube cancer, and peritoneal cancer, indications for first-line maintenance therapy with lucaparib were added (2A recommendation)[5]. The ATHENA study (NCT03522246) evaluated the efficacy of lucaparib first-line maintenance therapy, with 427 and 111 patients randomized to lucaparib and placebo groups, respectively. Among them, there were 185 and 49 HRD patients. The median progression-free survival (mPFS) for the intention-to-treat (ITT) population was 20.2 months vs. 9.2 months (HR=0.52, P<0.0001). However, there were significant differences in benefit among BRCAm, HRD, and HRP patients[6]. Lucaparib monotherapy for first-line maintenance therapy showed efficacy in the overall population, with statistical significance compared to placebo, but this indication has not yet received FDA approval[2]. The 2023 European Society for Medical Oncology (ESMO) guidelines mentioned this study without making a recommendation, and the European Medicines Agency (EMA) has not approved this indication[7-8].
ATHENA Study primary endpoint[6]
Maintenance therapy for recurrent ovarian cancer
Maintenance therapy for platinum-sensitive recurrent (PSR) ovarian cancer comprises a diverse category of indications. The FDA successively approved niraparib monotherapy on March 27, 2017, olaparib monotherapy on August 17, 2017, and lucaparib monotherapy on April 16, 2018, for PSR ovarian cancer maintenance therapy[2]. Based on data from key endpoint progression-free survival (PFS) in studies such as NOVA (NCT01847274), Study19 (NCT00753545), and ARIEL3 (NCT01968213), PARP inhibitor monotherapy is effective for the overall population with PSR ovarian cancer. Consequently, the initial indications do not require biomarkers as accompanying diagnostics. However, the results of these studies also indicate that the HRD population benefits more than the HRP population, with the highest benefit observed in patients with BRCA mutations within the HRD population[9-12].
As of October 1, 2020, in the gBRCA wild-type (gBRCA wt) cohort, niraparib compared to placebo showed a median overall survival (mOS) reduction of 5.4 months (31.1 months vs. 36.5 months, HR=1.1); similarly, non-gBRCAm/HRD-positive patients also exhibited a trend of impaired OS (37.3 months vs. 41.4 months, HR=1.32). In light of these results, the American Society of Clinical Oncology (ASCO) ovarian cancer PARP inhibitor treatment guidelines were rapidly updated, stating that for non-g/sBRCAm patients, the maintenance therapy with niraparib should weigh the potential PFS benefits against possible OS shortening (Type: evidence-based, net benefit; quality of evidence: low; strength of recommendation: moderate)[13]. On November 11, 2022, GSK announced the withdrawal of the maintenance therapy indication for niraparib in BRCA wt PSR patients, retaining the indication for gBRCAm PSR patients. This decision was reflected in the FDA label update on December 8, 2022[2]. The final results after a 7-year and 7-month follow-up showed an mOS of 40.9 months for the gBRCAm cohort in the niraparib group, compared to 38.1 months in the placebo group (HR=0.85); for the non-gBRCAm cohort, the mOS was 31.0 vs. 34.8 months (HR=1.06)[14].
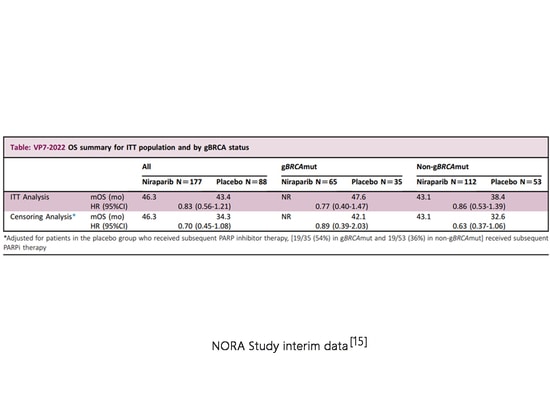
At the 2022 ESMO Virtual Congress, the mid-term overall survival (OS) data from the domestic phase III NORA study (NCT03705156) were presented. This is the first prospective phase III study in China investigating PARP inhibitors for maintenance therapy in PSR patients and the first and currently only study globally confirming OS benefits in the overall population with PSR maintenance therapy using a PARP inhibitor. In the intention-to-treat (ITT) population, the niraparib group showed an mOS of 46.3 months, extending 12 months compared to the placebo group after correction for crossover deletion. In the gBRCAm cohort, the niraparib group's mOS did not reach, with a placebo group mOS of 47.61 months. In the non-gBRCAm cohort, the niraparib group's mOS was 43.1 months, extending 10.5 months compared to the placebo group after crossover deletion[15]. The 2023 version of the ESMO guidelines cites this study but does not consider the results to be decisive[7]. As the European Medicines Agency (EMA) has not revoked the indication for non-gBRCAm patients, niraparib can still be used for the overall population with PSR, regardless of BRCA mutation status[7-8]. NMPA has also not revoked the indication for non-gBRCAm patients.
NORA Study interim data[15]
The results of the ARIEL3 study, presented at the 2022 International Gynecologic Cancer Society (IGCS) Annual Meeting, indicate that lucaparib did not demonstrate an overall survival (OS) benefit compared to placebo in both the intention-to-treat (ITT) population and the HRD-positive subgroup. In December 2022, CLOVIS ONCOLOGY INC filed for bankruptcy protection in the United States and withdrew the maintenance therapy indication for lucaparib in BRCA wt PSR patients while retaining the indication for BRCAm PSR patients[2]. Over a follow-up period of 8.2 years, the mOS in the ITT population was 36.0 months vs. 43.2 months[14]. In tBRCA mutated patients, the mOS in the lucaparib group was 45.9 months, compared to 47.8 months in the placebo group, with an HR of 0.83[2]. As the European Medicines Agency (EMA) has not revoked the indication for non-gBRCAm patients, the ESMO guidelines still recommend the use of lucaparib for the overall population with PSR, regardless of BRCA mutation status[7-8].
Olaparib's initial approval for maintenance therapy in PSR ovarian cancer was based on two studies, SOLO2 (NCT01874353) and Study19. The SOLO2 study included only gBRCAm patients, while Study19 included approximately half (136/265) BRCAm patients[16-17]. In the SOLO2 study, the olaparib group showed a significant mOS benefit compared to the placebo group, with 51.7 months vs. 38.8 months (HR=0.74, p=0.0537)[16]. In the Study19 study, the mOS for BRCAm patients was 34.9 months vs. 30.2 months (HR=0.62, p=0.02), while the mOS for BRCA wt patients was 24.5 months vs. 26.6 months (HR=0.83, p=0.37)[17]. Another single-arm OPINION study (NCT03402841) included non-gBRCA PSR patients who had previously received platinum-containing regimens in the second line and above. OS data presented at the 2022 ESMO Congress showed OS rates at 30 months for sBRCAm, HRD-positive including sBRCAm, HRD-positive excluding sBRCAm, and HRD-negative patients were 70.4%, 66.7%, 65.5%, and 38.9%, respectively, with an overall mOS of 32.7 months[18]. This is shorter than the data from the SOLO-2 study (51.7 months), suggesting that the genetic status influences the benefit of olaparib. On September 12, 2023, the FDA revoked the indication for olaparib in BRCA wt PSR patients for maintenance therapy but retained the indication for BRCAm PSR patients[2]. The NCCN guidelines have not been updated, but they have consistently recommended olaparib for BRCAm patients in the notes[5]. EMA approves olaparib for maintenance therapy in PSR ovarian cancer only for g/sBRCAm patients, and the ESMO guidelines make recommendations based on the approved scope[7-8]. The NMPA has not revoked the indication for non-BRCAm patients.
Orapali indication for maintenance treatment in BRCA wt PSR patients withdrawn[2]
Multiple lines of treatment for recurrent ovarian cancer
The treatment of recurrent ovarian cancer with multiple lines is the first indication for PARP inhibitors approved by the FDA. As early as December 19, 2014, the FDA approved olaparib for the fourth-line and beyond treatment of advanced ovarian cancer in patients with gBRCA mutations. Subsequently, on December 19, 2016, lucaparib was approved for the third-line and beyond treatment of patients with BRCA mutations, and on October 23, 2019, niraparib was approved for the fourth-line and beyond treatment of HRD-positive patients. However, these approvals were all based on data on objective response rate (ORR) and median duration of response (mDOR)[2].
The Phase III randomized controlled trial (RCT) ARIEL4 (NCT02855944) evaluated the efficacy of lucaparib compared to chemotherapy in BRCA-mutated recurrent patients who had previously received second-line or above chemotherapy. In the intention-to-treat (ITT) population, the mOS in the lucaparib group was 19.4 months, while in the chemotherapy group, it was 25.4 months (HR=1.31, p=0.0507), indicating a detrimental effect of lucaparib on OS. On June 10, 2022, the FDA revoked the approval of lucaparib for the third-line and beyond treatment of BRCA-mutated patients[13].
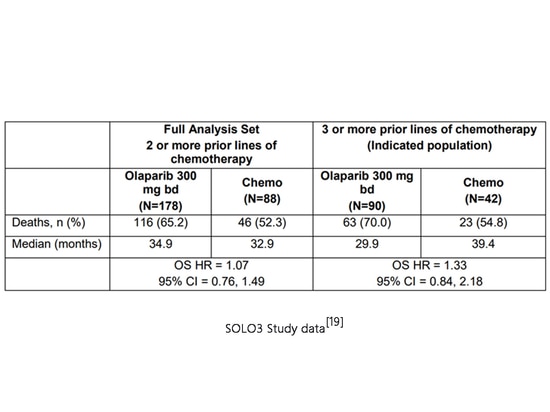
The Phase III SOLO3 study (NCT02282020) compared the efficacy of olaparib and non-platinum chemotherapy in gBRCA-mutated platinum-sensitive recurrent ovarian cancer patients who had received at least second-line platinum-based chemotherapy. As of April 16, 2021, the mOS in the olaparib group was 34.9 months, while in the chemotherapy group, it was 32.9 months (HR=1.07, p=0.71), showing no significant difference in OS. Subgroup analysis revealed potential survival detriment in patients who had received third-line or above chemotherapy, with an mOS of 29.9 months in the olaparib group compared to 39.4 months in the chemotherapy group (HR=1.33). AstraZeneca chose to selectively withdraw the indication for olaparib in the fourth-line and beyond treatment of advanced ovarian cancer patients with gBRCA mutations on August 26, 2022[13][19].
SOLO3 Study data[19]
Given the withdrawal of indications for late-line treatment by lucaparib and olaparib, GSK voluntarily withdrew the indication for niraparib in the fourth-line and beyond treatment of HRD-positive patients on September 14, 2022[13].
Although the FDA revoked the late-line treatment indications for the three PARP inhibitors mentioned above, the NCCN guidelines still retain recommendations for these three therapies, albeit with a lower level (category 3), even lower than the recommended level for unapproved therapies such as niraparib in combination with bevacizumab or pamiparib monotherapy (category 2B)[5]. EMA only approved lucaparib for the third-line and beyond treatment of BRCA-mutated patients and revoked this indication on August 12, 2022[8].
Other PARP inhibitors
Domestically produced fluzoparib and pamiparib, have not had recent updates to their indications. Fluzoparib is approved for maintenance therapy in PSR ovarian cancer, and both inhibitors for third-line and beyond treatment have entered medical insurance coverage, with gBRCAm mutations as a biomarker requirement for both late-line therapies[4]. Hengrui Medicine applied for the first-line maintenance therapy indication with the NMPA on August 23, 2023[20].
The PARP inhibitor veliparib, frequently mentioned in NCCN and ESMO guidelines, was evaluated in the VELIA study (NCT02470585). The study assessed the efficacy of veliparib in combination with carboplatin/paclitaxel for first-line chemotherapy and subsequent monotherapy for first-line maintenance therapy, with a total of 1140 patients included in the randomized groups. In the BRCAm cohort, the median progression-free survival (mPFS) was 34.7 months vs. 22 months (HR=0.44, p<0.001), in the HRD cohort it was 31.9 months vs. 20.5 months (HR=0.57, p<0.001), and in the ITT cohort it was 23.5 months vs. 17.3 months (HR=0.68, p<0.001). Overall survival (OS) data are still immature[21]. As of now, veliparib has not yet received approval from the FDA or EMA.
Summary
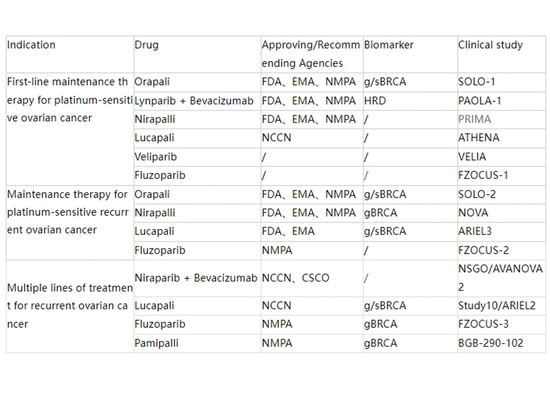
The table below summarizes the indications for PARP inhibitors in ovarian cancer to date.:
SpaceGen provides BRCA1/2 gene mutation testing, ovarian cancer 12 gene mutation testing, ovarian cancer 70 gene + MSI testing and homologous recombination repair deficiency (HRD) testing and other projects to serve ovarian cancer patients with different needs, suggesting PARP inhibitors efficacy.
References
[1] Clinical Application Guidelines for PARP Inhibitors in Ovarian Cancer
[2] FDA official website database
[3] N Engl J Med. 2019 Dec 19;381(25):2391-2402.
[4] National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalog (2022)
[5] NCCN Ovarian Cancer, Fallopian Tube Cancer, and Primary Peritoneal Cancer Diagnosis and Treatment Guidelines 2023 v2
[6] J Clin Oncol. 2022 Dec 1;40(34):3952-3964.
[7] Ann Oncol. 2023 Oct;34(10):833-848.
[8] EMA official website database
[9] N Engl J Med. 2016 Dec 1;375(22):2154-2164.
[10] Lancet Oncol. 2014 Jul;15(8):852-61.
[11] Lancet Oncol. 2016 Nov;17(11):1579-1589.
[12] Lancet. 2017 Oct 28;390(10106):1949-1961.
[13] J Clin Oncol. 2022 Nov 20;40(33):3878-3881.
[14] Clinical Trials official website database
[15] Ann Oncol. 2023;34(1):124-125.
[16] Lancet Oncol. 2021 May;22(5):620-631.
[17] Lancet Oncol. 2016 Nov;17(11):1579-1589.
[18] Annals of Oncology (2022) 33 (suppl_7): S235-S282.
[19] AstraZeneca: Dear healthcare professional letter. 2022.
[20] Official website of the Center for Drug Evaluation of the State Drug Administration
[21] N Engl J Med. 2019 Dec 19;381(25):2403-2415.