#Industry News
New targets are being discovered
New targets are being discovered
Background
The treatment of solid tumors has entered the era of targeted therapy and immunotherapy, with broad-spectrum inhibitors continuously approved both domestically and internationally. The detection of molecular markers indicating the effectiveness of broad-spectrum targeted and immunotherapy has become particularly important. With the advent of the era of precision tumor treatment, conventional pathological diagnostic methods can no longer meet clinical demands. Both domestic and international guidelines and consensus acknowledge the value of comprehensive molecular testing based on next-generation sequencing (NGS) in guiding clinical treatment. Comprehensive and accurate diagnosis of tumor genes has become a prerequisite for clinical diagnosis and treatment.
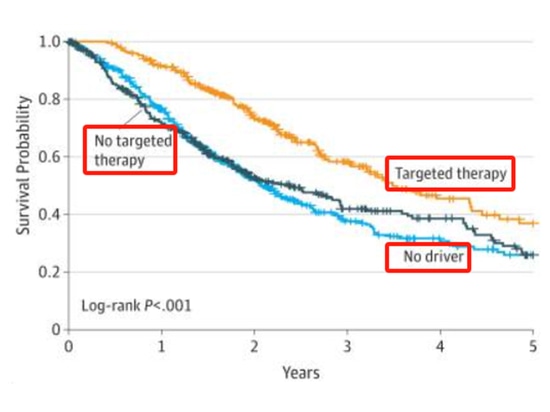
Taking lung adenocarcinoma as an example, research indicates [1] that about two-thirds of lung cancers have identifiable driver gene mutations. Lung cancer patients with driver gene mutations, if receiving corresponding precision targeted therapy, have a longer survival period compared to patients without gene mutations. Additionally, lung cancer patients with driver gene mutations, when undergoing precision targeted therapy compared to those not receiving targeted therapy, also experience a longer survival period.
Note: Median overall survival (95% CI): Carcinogenic driver + no targeted treatment, 2.38 (1.81-2.93); Carcinogenic driver + targeted treatment, 3.49 (3.02-4.33); No carcinogenic driver, 2.08 (1.84-2.46).
With the development of new targets and new drugs, more patients in the future will have the opportunity to choose new targeted treatment options with better efficacy and fewer side effects.
KRAS-G12D
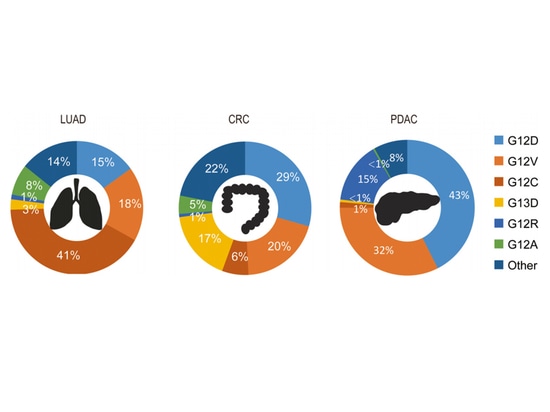
In terms of the KRAS-G12D mutation, it has the highest mutation rate in lung adenocarcinoma (LUAD), pancreatic ductal adenocarcinoma (PDAC), and colorectal cancer (CRC). The most common KRAS mutation in human cancers is KRAS-G12D, with a mutation frequency of approximately 33%, and it is the most prevalent KRAS allele mutation in PDAC (46%). With the introduction of MRTX1133, the development of small molecule inhibitors directly targeting KRAS-G12D has become possible.
▲Distribution of KRAS alleles in selected tumor types
KRAS-G12D Inhibitors Classification[2]
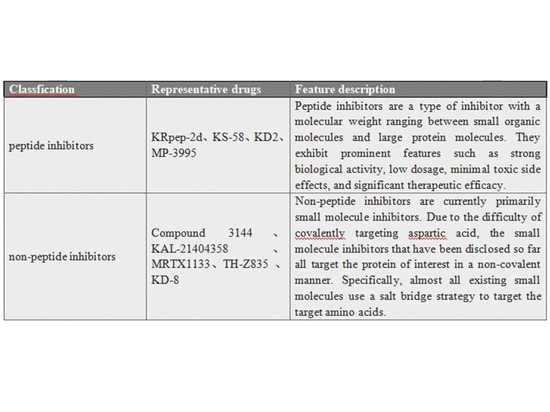
The KRAS-G12D mutation, which has the highest proportion in RAS mutations, is frequently found in malignant solid tumors. However, effective inhibitors are currently lacking. Existing preclinical inhibitors can be broadly categorized into peptide and non-peptide classes. Peptide inhibitors face challenges such as poor membrane permeability and drug-like properties. On the other hand, significant progress has been made in the development of small molecule inhibitors within the non-peptide category.
▲Classification and Characteristics of KRAS-G12D Inhibitors
NRG1
[3] NRG1 is a ligand that binds to HER3 and is frequently fused in lung cancer, pancreatic cancer, and other cancers. NRG1 rearrangements have also been found in non-small cell lung cancer and pancreatic cancer patients, leading to increased NRG1 expression, suggesting a carcinogenic role for NRG1. Additionally, NRG1 fusions are common in kidney cancer, thyroid cancer, gallbladder cancer, bladder cancer, ovarian cancer, pancreatic cancer, as well as sarcomas and neuroendocrine cancers, among other malignancies.
▲NRG1 fusion partner genes discovered in tumors of different organs[4]
MCLA-128: In 79 evaluable patients, 70% of patients experienced tumor shrinkage after baseline treatment[5].
The FDA has granted breakthrough therapy designation to zenocutuzumab (MCLA-128) for its potential therapeutic use in late-stage unresectable or metastatic NRG1 fusion-positive pancreatic cancer patients who have experienced disease progression after prior systemic therapy or lack satisfactory alternative options.
The data presented at the 2022 ASCO Annual Meeting revealed that, at a median follow-up of 6.3 months, the objective response rate (ORR) induced by zenocutuzumab was 34% (95% CI, 24%-46%), with up to 70% of patients experiencing tumor shrinkage. The ORR for pancreatic ductal adenocarcinoma (PDAC) patients was 42% (95% CI, 20%-67%).
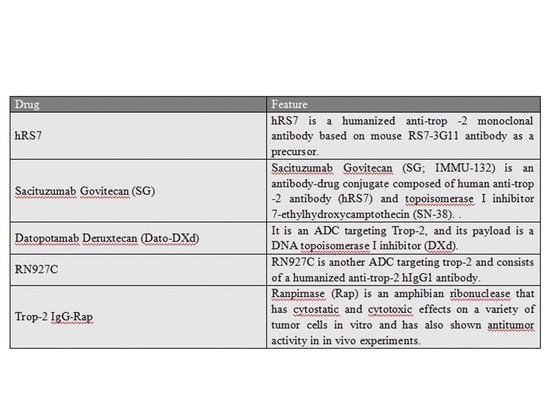
Trop-2
Trop-2 is also an adverse prognostic marker in various solid tumors such as breast cancer, ovarian cancer, prostate cancer, hepatocellular carcinoma, and oral squamous cell carcinoma. Specifically, Trop-2 overexpression is significantly associated with the prognosis of female reproductive and gastrointestinal tumors.
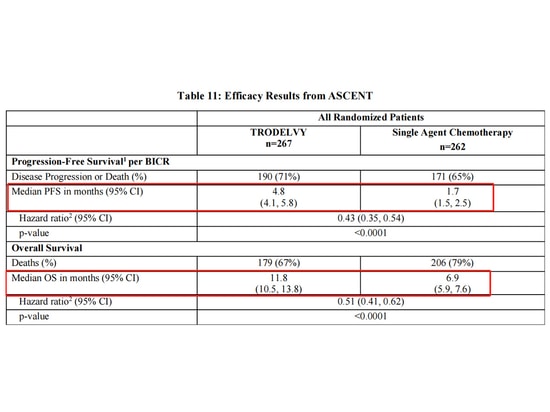
Trodelvy (sacituzumab govitecan) is the first and currently the only approved Trop-2-targeting antibody-drug conjugate (ADC) that has been marketed. It is used for locally advanced or metastatic triple-negative breast cancer, and experimental results comparing Trodelvy with monotherapy chemotherapy have been reported [6].
SG is currently being tested in several solid tumor clinical trials:
▲Representative SG-related clinical trials[7]
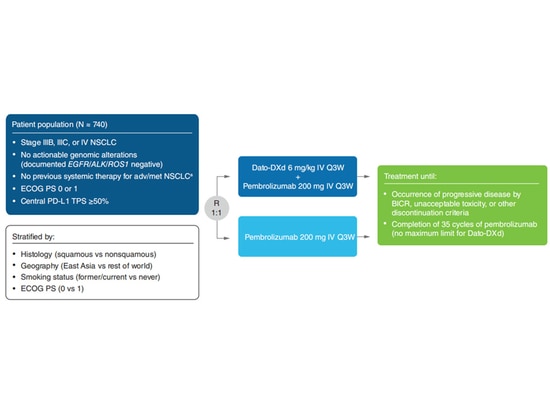
TROPION-Lung08: Phase III study of datopotamab deruxtecan + pembrolizumab as first-line treatment for advanced NSCLC: The objective response rate (ORR) of Dato-DXd in combination with pembrolizumab as first-line treatment is 62% (8/13 patients), with a disease control rate of 100% [8].
▲TROPION-Lung08 Study Design
Summary of related investigational drugs:
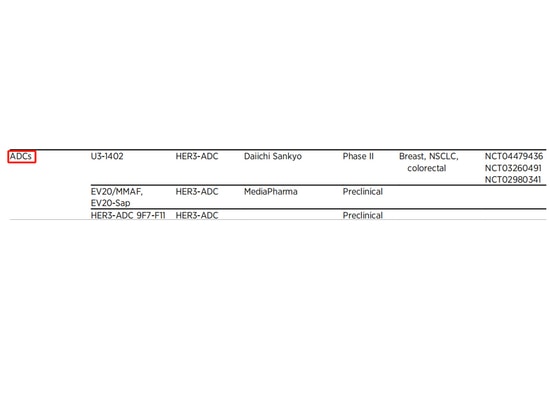
HER3
HER3 is the third member of the HER family, following EGFR (also known as HER1) and HER2. The overexpression of HER3 serves as a bypass mechanism for various targeted therapies, contributing to resistance to various treatment drugs. Interest in targeting HER3 persists, and new mechanisms and treatment strategies targeting HER3 continue to emerge, including HER3-directed antibody-drug conjugates (ADCs), which are actively being studied in a wide range of cancer research [9].
▲ADC drug research summary
Summary
In addition to the aforementioned emerging targets, there are many other important targets that can be utilized in the development of broad-spectrum anticancer drugs, each achieving different research advancements. With the continuous updates and developments in molecular diagnostic testing, the options for targeted therapies may become more comprehensive, continually providing treatment opportunities for cancer patients. This is expected to be one of the important future directions in cancer treatment.
References
[1]Kris MG, et al. JAMA. 2014 May 21;311(19):1998-2006.
[2] Current research status of KRAS-targeted G12D inhibitors
[3]www.oncokb.org/gene/NRG1
[4]Genes Chromosomes Cancer. 2021;60:474–481
[5]https://www.onclive.com/view/fda-grants-breakthrough-therapy-designation-to-zenocutuzumab-for-nrg1-pancreatic-cancer
[6]FDA official website
[7]doi.org/10.1016/j.bbcan.2023.188902
[8]Future Oncol. (2023) 19(21), 1461–1472
[9]Clin Cancer Res 2021;27:3528–39
Statement: This article is for sharing only. If there are any copyright issues, please contact us as soon as possible and we will correct it as soon as possible. Thank you!
![▲NRG1 fusion partner genes discovered in tumors of different organs[4]](https://img.medicalexpo.com/images_me/projects/images-om/49306-19305662.jpg)

![▲Representative SG-related clinical trials[7]](https://img.medicalexpo.com/images_me/projects/images-om/49306-19305671.jpg)