#Industry News
Cell rep:Selenium intake can effectively prevent acute myeloid leukemia
Cell rep:Selenium intake can effectively prevent acute myeloid leukemia
Background
Acute Myeloid Leukemia (AML) is a malignant tumor characterized by abnormal proliferation of hematopoietic progenitor cells and stem cells infiltrating the bone marrow, blood, and other tissues. The cytogenetic and molecular heterogeneity contributes to the refractory nature and relapse of AML. A key obstacle in treatment is minimal residual disease (MRD), characterized by the presence of Leukemic Initiating Cells (LICs), which are crucial for disease relapse to some extent. These cells exhibit similarities with normal hematopoietic stem cells in terms of self-renewal, proliferation, and differentiation. Consequently, as cancer stem cell components are better defined, alternative therapies targeting LICs open new avenues for treating AML and other malignancies.
GPR44 is expressed in various cell types but is predominantly found in Th2 effector cells. Existing literature on GPR44 primarily focuses on its role in autoimmune diseases and type 2 immunity, with a noticeable lack of research on its role in hematologic malignancies.
In this study, the authors discovered that activating GPR44 could mediate endogenous anti-leukemic effects. Specifically, adding selenium to LICs from a mouse AML model and patient-derived AML cells resulted in the production of cyclopentenone prostaglandins (CyPGs). CyPGs, upon GPR44 activation, inhibit the KRAS-mediated MAPK and PI3K/AKT/mTOR signaling pathways, promoting apoptosis in AML cells. These findings underscore the significant therapeutic role of GPR44 in leukemia and provide a mechanistic basis for the chemopreventive properties of selenium and CyPGs in AML.
Research result
1.Supplementing selenium can improve the prognosis of acute myeloid leukemia (AML).
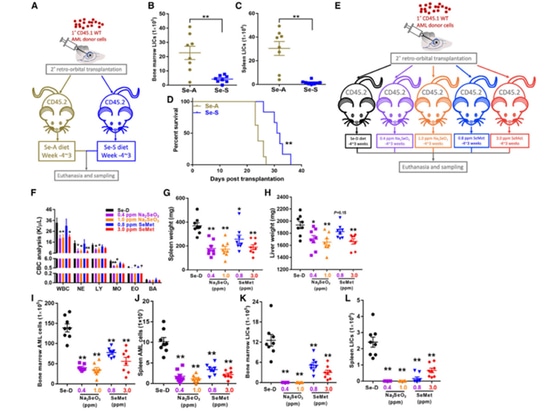
The author first transplanted CD45.1 WT AML cells into CD45.2 recipient mice that had been fed with either Se-A or Se-S for 4 weeks, creating secondary AML mice. After 3 weeks, the mice were sacrificed, and blood, bone marrow, and spleen specimens were collected. It was observed that Se-S significantly reduced the leukemia-initiating cell (LIC) burden in the bone marrow and spleen, prolonging the survival of AML mice. Subsequently, the effects of different forms of dietary selenium (including Na2SeO3 and SeMet) on AML mice were compared, revealing that dietary selenium could reduce the levels of white blood cells. It also alleviated spleen and liver enlargement. The author's research results confirm that supplementing selenium in the diet can effectively inhibit AML, and Na2SeO3 has a preventive advantage over SeMet in AML.
Figure 1. Se supplementation improves the outcome of AML
2.Selenium supplementation induces the production of endogenous CyPGs in AML.
Subsequently, the authors measured the levels of 15d-PGJ2 (prostaglandin J2) in the serum of AML mice and assessed the expression of cyclooxygenase-1 (COX-1) and hematopoietic PGD synthase (H-PGDS). The results showed that a selenium-enriched diet significantly enhanced the expression of the relevant proteins COX-1 and H-PGDS, promoting an increase in PGJ2 levels.
To further investigate the potential role of H-PGDS, the authors treated Se-S AML mice with the H-PGDS inhibitor HQL79 to block the production of endogenous CyPGs. The results showed that HQL79 treatment increased tumor burden and elevated the content of LICs in the bone marrow and spleen of Se-S mice. In conclusion, these results suggest that selenium supplementation mitigates AML by enhancing endogenous CyPGs through increased H-PGDS expression.
Figure 2. Se supplement induces endogenous production of CyPGs in AML
3.The activation of GPR44 can reduce the severity of leukemia
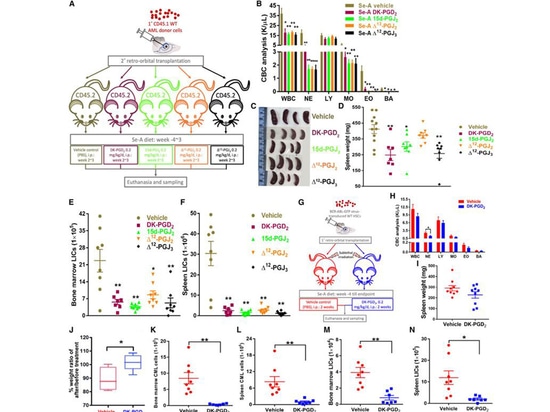
Subsequently, the authors tested whether different forms of exogenous CyPGs could treat AML in Se-A mice. The results showed that treatment with CyPGs (DK-PGD2, 15d-PGJ2, Δ12-PGJ2, and D12-PGJ3) reduced CBC in mice and slowed splenomegaly, except for DK-PGD2, all other compounds induced the death of human acute myeloid leukemia cells (MOLM13) in a dose and time-dependent manner. Additionally, in MOLM13 cells, the expression of GPR44 was detected at both the transcript and protein levels, with a significant increase in its intracellular form. This emphasizes the importance of CyPGs in the cell membrane localization of GPR44 in AML.
The authors further validated clinical relevance by treating patient-derived AML cells with DK-PGD2 or 15d-PGJ2. Among 12 AML patient samples, 9 responded to DK-PGD2, showing enhanced cell apoptosis. Examination revealed that patients sensitive to DK-PGD2 treatment had higher expression of GPR44, more than four times higher than refractory samples, further highlighting the crucial role of GPR44 in AML. These results indicate that increasing endogenous CyPGs or using pharmacological doses of exogenous CyPGs effectively treats myeloid leukemia.
Figure 3. GPR44 activation decreases the severity of leukemia
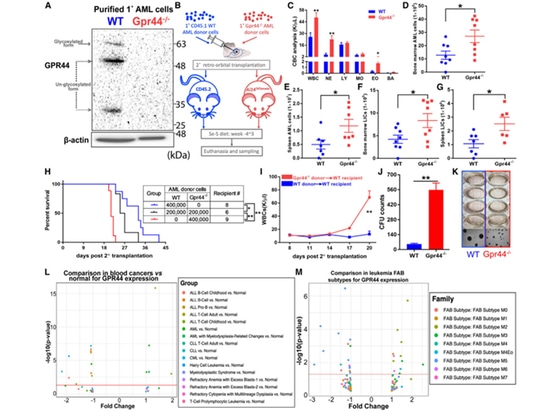
4.The absence of GPR44 in AML cells results in aggressive diseases. Previous studies have indicated that GPR44 plays a crucial role in CyPG-induced apoptosis of AML cells. Therefore, the authors validated the effects of GPR44 deficiency on AML cells. Compared to the control, transplantation of GPR44 - / - AML cells with Se-S diet led to an increase in WT receptor white blood cells and enlarged spleens. In mice transplanted with GPR44 - / - AML cells, the total number of Lin - AML cells and LICs in the bone marrow and spleen increased more than twofold compared to mice transplanted with WT AML. The loss of GPR44 resulted in a tenfold increase in the colony-forming ability of AML cells, suggesting that high expression of GPR44 may be a target for CyPG therapy rather than a prognostic factor.
Figure 4. Lack of GPR44 in AML cells results in aggressive disease
5. PPARγ Activity Influences the Progression of Gpr44−/− AML
Because PPARγ and GPR44 share the same ligands, the authors investigated the potential crosstalk between PPARγ and GPR44. Se-S mice with Gpr44 - / - AML cells were treated with the PPARγ selective antagonist GW9662, resulting in significantly higher CBC compared to mice treated with the control agent, accompanied by splenomegaly. In contrast, Se-S mice with Gpr44 - / - AML treated with rosiglitazone (a PPARγ agonist) showed a decrease in white blood cell levels and splenomegaly. Overall, these data suggest that AML cells lacking GPR44 expression can still be partially affected by Se or targeted CyPGs through PPARγ signaling, leading to cell death possibly through P53 activation. However, the expression of activated GPR44 results in a stronger response to free AML LICs.
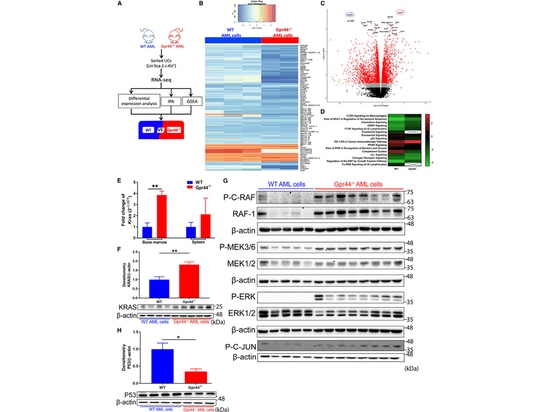
6.Lack of GPR44 Activation Affects KRAS-MAPK Signaling in AML Cells
Subsequently, the authors conducted RNA sequencing analysis on WT and GPR44 - / - AML LICs and found significant changes in the transcriptional program due to GPR44 deficiency. Additionally, both mRNA and protein expression of KRAS were significantly increased in GPR44 - / - AML cells. Enhanced phosphorylation of MAPK components (RAF, MEK, and ERK) was observed in GPR44 - / - AML cells, accompanied by increased expression of RAF-1. The downstream effect of MAPK signaling, increased phosphorylation of C-JUN, further confirmed the activation of MAPK signaling in GPR44 - / - AML cells, consistent with the role of KRAS-MAPK signaling in leukemia development. This provides further explanation for the increased invasiveness of GPR44 - / - AML cells.
Figure 5. Lack of GPR44 activates KRAS-MAPK signaling in AML cells
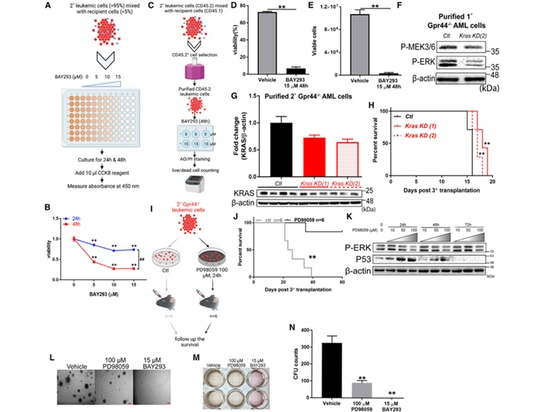
7.Inhibition of KRAS/MAPK signaling reverses the impact of GPR44 knockout on AML.
The author utilized methods such as the KRAS inhibitor BAY293, lentivirus expressing dCas9KRAB33, and specific gRNA targeting the mouse KRAS promoter for validation. The results demonstrated a significant decrease in the viability of GPR44 - / - AML cells, along with a notable reduction in the expression of anti-apoptotic genes and cell cycle-related genes. The survival rate of recipient mice also significantly increased.
Subsequently, the author treated GPR44 - / - AML cells with the MEK-selective inhibitor PD98059 in vitro, leading to an increased survival rate in transplanted mice. PD98059-treated GPR44 - / - AML cells exhibited decreased expression of P-ERK, increased expression of P53, and weakened expression of anti-apoptotic genes, cell cycle-related genes, and stem cell quiescence genes. Inhibiting KRAS or MEK impaired the colony-forming ability of GPR44 - / - AML cells.
In summary, these data demonstrate a certain connection between GPR44 and the regulation of KRAS/MAPK signaling. This may serve as an alternative target for reversing AML deterioration in the absence of GPR44."
Figure 6. Inhibition of KRAS/MAPK signaling reverses GPR44 KO-associated effect in AML
8.The loss of GPR44 exacerbates AML by activating the PI3K/AKT/mTOR pathway.
KRAS is involved in the PI3K/AKT/mTOR signaling pathway. Therefore, the authors conducted an evaluation in WT (wild-type) and GPR44 - / - (GPR44 knockout) AML cells. In GPR44 - / - AML cells, the expression and phosphorylation levels of PI3K and AKT were significantly upregulated. Additionally, the expression of PKA and PKC increased. The sharp decrease in PTEN and P-PTEN suggests that the loss of GPR44 may have a negative impact on the tumor-suppressive role of PTEN in AML. The use of PI3K inhibitors (LY294002 and Wortmannin) led to a decrease in viability and inhibited colony-forming ability in GPR44 - / - AML cells, indicating that PI3K is highly expressed and activated in these cells.
Summary of the Article
In this study, the supplementation of dietary selenium with GPR44 in a human leukemia mouse model, human AML cell lines, and patient samples resulted in the apoptosis of AML LICs (Leukemia-Initiating Cells) triggered by endogenous CyPGs produced through the COX-H-PGDS pathway or exogenous CyPGs activation. In AML mice, the absence of GPR44 exacerbated the disease through the upregulation of RTK-related KRAS-mediated MAPK and PI3K/AKT/mTOR signaling pathways. CyPGs did not affect normal hematopoietic stem cells in the bone marrow of AML or CML mice, making it a safe therapeutic option that ensures effective recovery of normal hematopoietic function during leukemia.