#Industry News
Fully automated library preparation builds a new matrix for companion diagnostics of tumors
Fully automated library preparation builds a new matrix for companion diagnostics of tumors
The first consensus on "Automation and Standardization of Next-Generation Sequencing in Clinical Practice", led by the Medical Laboratory Engineering Branch of the Chinese Society of Biomedical Engineering, was published in the "Chinese Journal of Biomedical Engineering" at the end of 2023. With the development of tumor companion diagnostics and the improvement of library preparation automation, Feishuo Biotech, as a molecular diagnostic enterprise integrating R&D, production, and sales of in vitro diagnostic reagents, has developed a series of molecular detection products for tumor diagnosis, typing, monitoring, and screening based on the NGS technology platform. Actively following the consensus, Feishuo Biotech has launched a dedicated automated solution for NGS library preparation, efficiently realizing the automation and standardization of processes such as nucleic acid sample preparation, library preparation, and library quality control, further improving the gene testing application chain, enhancing overall strength and competitiveness in the user's overall solution.
Qualifications and Patent Technologies
The company has a strong research and technology team, equipped with high-throughput sequencing platforms, digital PCR platforms, fluorescent quantitative PCR platforms, nucleic acid mass spectrometry platforms, etc. Based on this, it has independently developed more than 40 products, applied for 41 domestic patents, 3 international PCT patents, obtained 7 invention patents, 2 utility model patents, and 12 software copyrights.
Product reagent kit
The company possesses advanced facilities and international standard GMP workshops, with a sound quality management system in place and certified by the TUV ISO13485 medical device quality management system of South Germany. Currently, several reagent kits have obtained CE-IVD certification and NMPA certification, with several others in the process of certification registration.
Class I medical device filing certificate, gene sequencing library reagent kit
International EU CE-IVD certification
Human tumor multi-gene mutation detection reagent kit (semiconductor sequencing method), human BRCA1/2 gene mutation detection reagent kit (high-throughput sequencing method), human radiotherapy-related gene SNP detection reagent kit (high-throughput sequencing method), human drug genome SNP detection reagent kit (high-throughput sequencing method), and so on.
Class III medical device registration certificate
Lung cancer NGS multi-gene combined detection product "Human EGFR/KRAS/BRAF/HER2/ALK/ROS1 gene mutation detection reagent kit (semiconductor sequencing method) (National Medical Products Administration Registration No. 20203400094)", human EGFR gene mutation detection reagent kit (multiplex fluorescence PCR method) (National Medical Products Administration Registration No. 20193400366), human K-ras gene mutation detection reagent kit (multiplex fluorescence PCR method) (National Medical Products Administration Registration No. 20223400275), and so on.
Fully automatic library preparation and evaluation machine
To improve the efficiency of companion diagnostics, our company uses the Human EGFR/KRAS/BRAF/HER2/ALK/ROS1 gene mutation detection reagent kit (semiconductor sequencing method) based on NGS fully automatic library preparation and evaluation machine for evaluation and constructs an expanded high-throughput automated tumor companion diagnostic testing platform application. To comprehensively improve the quality and efficiency of the testing process, the automation process of this platform is optimized with module design and upgraded bench layout to match different library preparation throughputs, significantly improving library preparation efficiency. With more than 90% reduction in manual operations while ensuring high-quality library preparation, it frees up the hands of laboratory personnel, improves work efficiency, effectively reduces and avoids the possibility of human errors, thereby ensuring precise, reliable, stable, and efficient results.
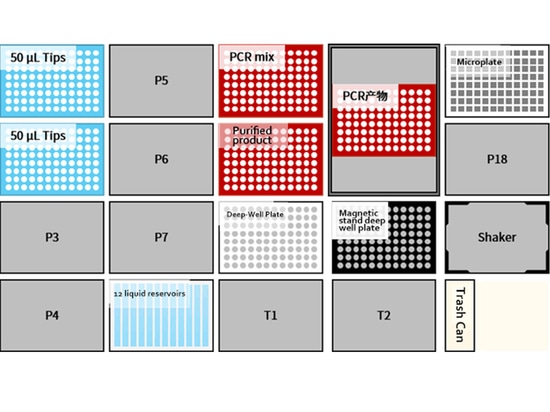
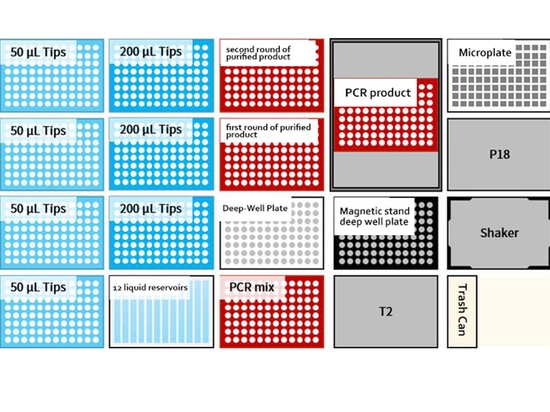
Countertop layout
01 Low throughput: 8 samples/time
02 Medium and high throughput: 48 samples/time
Quality analysis
Quantitative analysis of library concentration shows that the uniformity of automated library preparation is superior to manual operation. The peak graph of library fragment analysis shows a clear main peak with no impurities. Capillary electrophoresis results show that the main peak range of the library is 200-300 bp.
In the future, our company will further expand the application and development of genetic testing products on automated library preparation platforms, construct a new matrix for tumor companion diagnostics, enrich the application value of precision diagnosis and treatment, and better provide high-quality precision diagnosis services for a wide range of tumor patients.