#Industry News
Navient Receives Health Canada Approval for the Spine application
Navient Receives Health Canada Approval for the Spine application
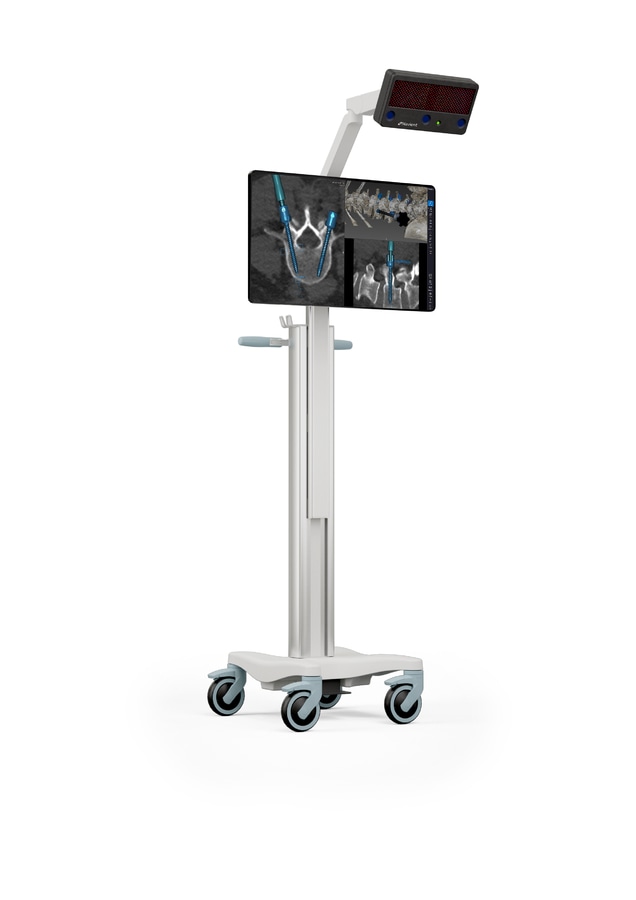
ClaroNav Kolahi Inc (CKI) is pleased to announce that it has received Health Canada approval for its Navient Spine application as a Class III medical device. Navient has been previously approved by Health Canada for the Cranial and ENT applications.
"Navient is one of the very few image guided navigation systems approved by Health Canada as a Class III device. Upgrading our license to a higher class demonstrates our deep commitment to product quality and safety.” said Ahmad Kolahi, CEO of CKI “Navient offers an elegant hardware and intuitive software. It is the most compact and potable navigation system in the market. Its instruments are steam sterilizable, and there is no disposable cost per procedure. Our primary goal is to offer an intuitive, accurate and affordable product and make it standard-of-care around the world."
Navient is currently installed in over 130 sites in 40 countries and approved by the regulatory bodies in many countries including, European CE (for ENT, Cranial and Spine application), US FDA (for ENT application), Chinese MNPA (ENT & Cranial application. Spine pending), Taiwan, Brazil and Korea (for ENT application), Peru, Colombia, Turkey, Thailand, Indonesia, Honduras, Guatemala, Ecuador, Philippines, UAE, …