#Industry News
Tow's WBP Empowers Overseas Research: The Latest Progress in the Treatment of Asthma Caused by Mugwort Allergy!
A study in npj Vaccines compared rArt v 1-based SLIT and SCIT in asthma mice. It covered design, results and WBP system used.
Research Article Published in npj Vaccines on April 2nd
Title: "Comparison of rArt v 1-based sublingual and subcutaneous immunotherapy in a murine model of asthma"
Authors: Tabynov, K., Tailakova, E., Rakhmatullayeva, G. et al.
This study compared the efficacy and immune mechanisms of sublingual immunotherapy (SLIT) and subcutaneous immunotherapy (SCIT) using recombinant mugwort major allergen Art v 1 (rArt v 1) in a murine asthma model. BALB/c mice were sensitized and treated with rArt v 1 SLIT, rArt v 1 SCIT (adjuvanted with ISA-51), a commercial mugwort pollen extract SLIT, or PBS (control). Key findings include:
Efficacy: Both therapies improved lung pathology and reduced airway hyperresponsiveness, but SCIT outperformed SLIT in suppressing late-phase allergic reactions (e.g., ear swelling) and lung inflammation.
Immune Mechanisms:
SCIT induced stronger Th1-polarized responses (e.g., elevated IFN-γ, increased IgG2a/IgG1 ratios).
SLIT enhanced mucosal IgA production but retained Th2 bias (IgG1 dominance).
Safety: Recombinant rArt v 1 SLIT did not elevate total IgE levels, whereas the commercial SLIT vaccine increased sensitization risk.
Innovation: First systematic comparison of SCIT and SLIT using a single recombinant allergen in mice, demonstrating cross-protection against natural pollen extracts.
Experimental Methods
1. Cloning, Expression, and Purification of Recombinant Art v 1
Gene Cloning: The optimized Art v 1 gene (GenBank ID PQ223694.1) was inserted into the pET28b vector using NdeI/XhoI restriction sites.
Expression and Purification: Expressed in E. coli C41, induced with IPTG, and purified via metal-affinity chromatography. SDS-PAGE and Western blot confirmed protein expression. Endotoxin removal yielded >95% pure protein with <5 EU/mL endotoxin.
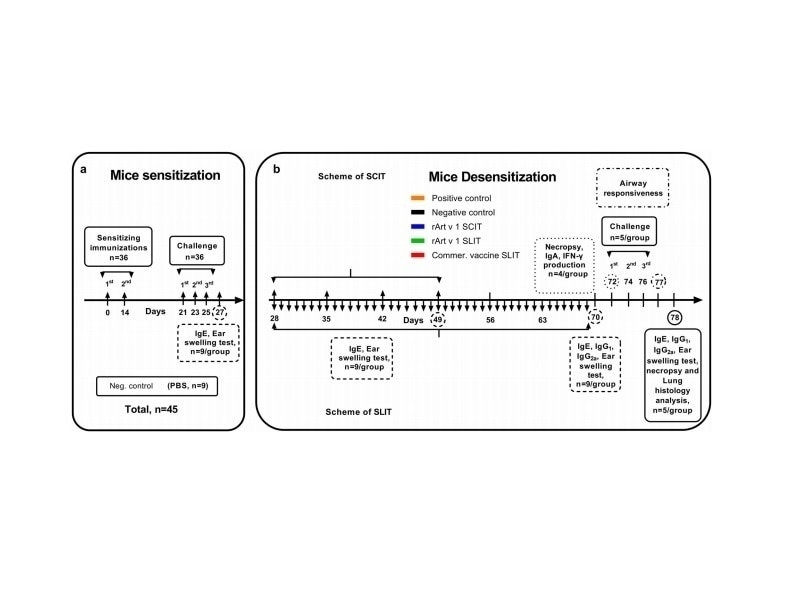
2. Mouse Sensitization and Asthma Model
Sensitization: BALB/c mice were intraperitoneally injected with aluminum hydroxide-adsorbed mugwort pollen extract (1000 PNU) twice at 14-day intervals.
Asthma Induction: Allergen challenges via inhalation and intranasal instillation of pollen extract. PBS was used for the negative control.
3. Immunotherapy Protocols
SCIT: Subcutaneous injections of rArt v 1 + ISA-51 adjuvant (4 weekly doses, cumulative 22 μg/mouse).
SLIT: Daily sublingual administration of rArt v 1 (0.001–10 μg, 42 days; cumulative 148 μg/mouse) or commercial pollen extract.
Controls: Positive (PBS-treated sensitized mice) and negative (non-sensitized mice).
4. Immunological and Pathological Assessments
Antibody Levels: Total IgE, Art v 1-specific IgG1, IgG2a, and IgA measured by ELISA.
Cellular Immunity: IFN-γ production in splenocytes post rArt v 1 stimulation.
Ear Swelling Test: Auricle thickness measured after allergen challenge.
Airway Hyperresponsiveness: Methacholine-induced airway resistance assessed using Tow’s WBP-M system (Penh values).
Lung Histopathology: H&E staining scored peribronchial inflammation, eosinophil infiltration, and goblet cell metaplasia (0–7 scale).
Experimental Results
1. Cloning, expression, purification, and analysis of rArt v 1 protein. (Figure 1)
Recombinant rArt v 1 was successfully expressed in E. coli C41, with a major band at ~23 kDa on SDS-PAGE (lower than natural glycosylated Art v 1).
Western blot confirmed reactivity with anti-His tag and natural Art v 1 polyclonal antibodies, confirming proper folding.
Figure 1
2. Inter- and intra-group- as well as time-wise comparison of total IgE levels. (Figure 2)
SCIT rapidly and stably reduced IgE levels, approaching non-sensitized controls. SLIT showed weaker IgE suppression.
Commercial SLIT increased sensitization risk due to natural allergens.
Figure 2
3. Comparison of allergen-specific IgG1, IgG2a and IgA and IFN-γ production levels in the different mouse groups. (Figure 3)
SCIT drove Th1 polarization (high IgG2a, IFN-γ), while SLIT promoted mucosal IgA (Th2 bias).
Combined Th1 (SCIT) and mucosal (SLIT) pathways may enhance therapeutic efficacy.
Figure 3
4. Effects of different forms of ASIT on allergen-induced ear swelling, airway responsiveness and allergen-induced lung inflammation.(Figure 4)
Ear Swelling: SCIT fully suppressed swelling post-treatment, unlike SLIT or commercial vaccine.
Airway Resistance: All ASIT groups reduced Penh values to near-negative control levels.
Lung Pathology: SCIT minimized inflammation (score: 1.6/7), while SLIT and commercial SLIT showed moderate scores (3.2–3.8).
Figure 4
Tow-Int Tech Whole Body Plethysmography
During the experiment, researchers utilized Tow's Whole Body Plethysmography (WBP) system to assess airway hyperresponsiveness (AHR) in mice. The results demonstrated that all ASIT groups (SCIT, SLIT, and commercial vaccine) exhibited a significant reduction in airway resistance (Penh values), reaching levels comparable to the negative control group. This outcome provided robust data support for the experimental conclusions.

The WBP system employs unrestrained whole-body plethysmography, enabling pulmonary function and airway reactivity testing in conscious, freely moving small animals. During measurement, the animal's breathing induces thoracic movements, which alter the chamber volume. These volumetric changes are detected by a pressure transducer, converted into electrical signals via an amplifier, and processed by computer software to generate real-time respiratory waveforms and calculate key parameters, including:
Tidal Volume (Vt)
Peak Expiratory Flow (PEF)
Respiratory Frequency
Enhanced Pause (Penh).
Customer Appreciation | Congratulations to Prof. Kairat Tabynov's Team on Publishing in npj Vaccines
On the day their research was officially published in npj Vaccines, we were honored to receive a letter of gratitude from Prof. Kairat Tabynov’s research group. The team highly commended the precision and reliability of Tow’s WBP system in airway hyperresponsiveness (AHR) testing and expressed appreciation for the professional support provided by our technical team.
We sincerely wish Prof. Kairat Tabynov’s team continued breakthroughs in the field of allergen immunotherapy and look forward to their future contributions of globally impactful innovations! We are eager to deepen collaboration with the research group to jointly advance the clinical translation of precision medicine technologies.
References:
[1]Tabynov, K., Tailakova, E., Rakhmatullayeva, G. et al. Comparison of rArt v 1-based sublingual and subcutaneous immunotherapy in a murine model of asthma. npj Vaccines 10, 66 (2025). https://doi.org/10.1038/s41541-025-01112-1
Contact us now!
We are committed to making your research easier, more accurate, and more efficient and helping you build confidence in your data! We have provided services for a large number of customers and have rich experiences in offering customized, professional solutions according to your needs.