#Product Trends
Optogenetics and whole-body plethysmography
to explore mechanisms behind respiratory rhythm control
Introduction
The study titled "Dbx1 Pre-Bötzinger Complex Interneurons Comprise the Core Inspiratory Oscillator for Breathing in Unanesthetized Adult Mice" by Nikolas C. Vann et al., published in eNeuro, investigates the mechanism by which Dbx1-derived pre-Bötzinger Complex (preBötC) neurons generate respiratory rhythms in conscious adult mice. By employing optogenetic techniques, the researchers precisely manipulated the activity of Dbx1 preBötC neurons expressing archaerhodopsin (a light-sensitive inhibitory protein) or channelrhodopsin (a light-sensitive excitatory protein) and evaluated the impact on breathing behavior.
Research Background
Inspiratory breathing movements in mammals originate from the rhythmic neural activity in the brainstem’s pre-Bötzinger Complex (preBötC). However, the precise neuronal composition of the preBötC remains unclear. Dbx1-derived neurons are thought to play a significant role in generating respiratory rhythms. These neurons exhibit rhythmic discharges in perinatal mice, but their role in adult mice has not been definitively established due to their involvement in motor pattern control and non-respiratory functions.
Experimental Methods
Experimental Animals
Female Dbx1CreERT2 mice, expressing tamoxifen-sensitive Cre recombinase in Dbx1-derived progenitor cells, were crossed with mice carrying various reporter genes. Neuronal expression was induced using tamoxifen treatment, with wild-type littermates serving as controls. All experiments were approved by the institutional animal care and use committee and adhered to relevant policies and guidelines.
Brain Slice Preparation and Recordings
Neonatal Dbx1;ArchT mice were anesthetized by hypothermia and decapitated. Brain slices containing the preBötC were prepared and perfused in a recording chamber with elevated potassium ion concentration. Inspiratory motor outputs were recorded, and whole-cell patch-clamp recordings were used to detect changes in neuronal membrane potentials.
Viral Injection and Optical Fiber Implantation
Adult Dbx1;ArchT and Dbx1;CatCh mice were anesthetized and underwent craniotomy for viral injections and optical fiber implantation. Dbx1;CatCh mice received virus injections to induce recombination prior to fiber implantation. Following recovery, experiments were conducted.
Respiratory Measurements
Respiratory behavior was measured using whole-body plethysmography. Mice were lightly sedated with ketotifen or briefly anesthetized with 2% isoflurane. Respiration was assessed by measuring airflow signals through a pressure circuit and differential pressure transducer.
Optogenetic Manipulation
Mice were exposed to light pulses of varying intensities (6.8, 8.6, or 10.2 mW) and durations (5 seconds or 100 milliseconds) delivered at intervals of at least 30 seconds. Multiple stimulations were performed on each mouse, and changes in respiratory frequency, tidal volume, minute ventilation, and phase resetting effects were analyzed.
Data Analysis
Respiratory parameters were analyzed using plethysmography software. Paired t-tests and other statistical methods were used to compare parameters under different conditions. Phase response curves were plotted by grouping and averaging the respiratory phase effects of light pulses.
Histological Examination
Post-experiment, animals underwent perfusion and tissue fixation. Brain sections were stained with NeuroTrace and examined under brightfield and confocal microscopy to verify fiber tip placement and adjust image contrast.
Research Findings
Optogenetic Activation (Dbx1;CatCh Mice)
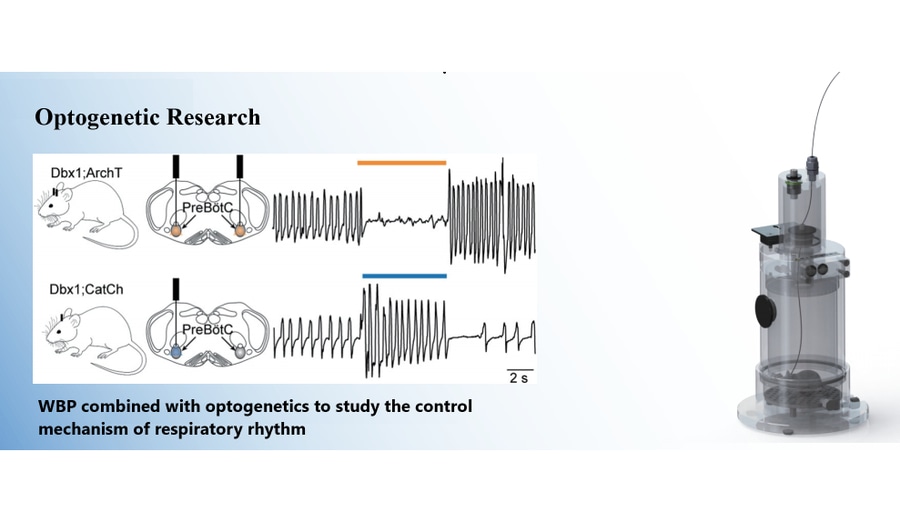
Light stimulation of the preBötC increased respiratory frequency in both anesthetized and awake states but did not significantly affect tidal volume or minute ventilation. Wild-type littermates showed no changes. Short-duration light stimulation during the inspiratory phase prolonged inspiratory time and delayed subsequent breaths (phase delay). During inspiratory-expiratory transition or expiratory phases, stimulation advanced subsequent breaths (phase advance).
Optogenetic Inhibition (Dbx1;ArchT Mice)
Light inhibition of the preBötC reduced respiratory frequency, tidal volume, and minute ventilation in both anesthetized and awake states, with higher light intensity leading to greater effects, including respiratory arrest. Wild-type littermates showed no changes. Short-duration light inhibition during early inspiration advanced the next inspiration (phase advance) and shortened inspiratory time. In early expiration, it delayed the next inspiration (phase delay).
Electrophysiological Findings
In neonatal Dbx1;ArchT mouse brain slices, 589-nm light caused hyperpolarization of Dbx1 preBötC neurons, suppressing rhythm generation and motor outputs. Hyperpolarization effects were predominantly direct postsynaptic and minimally affected non-Dbx1 neurons.
Discussion
Rhythm and Pattern Generation
Sustained light inhibition reduced respiratory frequency and even caused respiratory arrest, indicating that Dbx1 neurons are integral to the core oscillator. The reduction in tidal volume suggests their role in motor pattern control. Sustained light stimulation increased frequency significantly, differing from other studies due to experimental condition variations.
Phase Resetting Experiments
Both short-duration activation and inhibition affected respiratory phase and inspiratory time across cycles, supporting the view that Dbx1 preBötC neurons are part of the inspiratory rhythm generator.
Exclusion of Non-Specific Effects
Experimental design ruled out contributions from preBötC input perturbations, axonal terminals, or passing axons, further corroborating the role of Dbx1 preBötC neurons in rhythm generation.
Role of Glial Cells
Glial cells were unlikely to influence the results of inhibition experiments. While CatCh expression in glial cells might have contributed to activation experiments, comparison of ArchT and CatCh effects suggested a dominant neuronal role.
Core Oscillator Size
Although most Dbx1 preBötC neurons serve non-rhythmic functions, a subset forms the inspiratory core oscillator. Future studies are needed to quantify the proportion of non-Dbx1-derived rhythmogenic neurons and distinguish between rhythmogenic and non-rhythmogenic Dbx1 neurons.
Conclusion
This study reveals that Dbx1 preBötC neurons directly influence respiratory frequency and timing, establishing them as a core component of the inspiratory oscillator. These findings enhance our understanding of respiratory physiology, with implications for studying respiratory disorders and developing therapeutic targets.
Equipment Used in the Study
Whole-body Plethysmography System
Used to measure respiratory behavior in freely moving animals. The system detects volume changes caused by thoracic movement, converting pressure signals into electrical signals for respiratory curve analysis and parameter calculation (e.g., tidal volume, peak expiratory flow, respiratory rate).
Optogenetic Equipment
The 589-nm laser (Dragon Lasers) and the 473-nm laser (Dragon Lasers) were used to connect optical fibers for Dbx1;ArchT and Dbx1;CatCh mice, respectively, enabling optogenetic operations.
Tow-Int Tech Whole-Body Plethysmography System
Designed for respiratory function and airway responsiveness testing in conscious, unrestrained animals. By combining optogenetics with advanced respiratory measurements, the system facilitates research into respiratory rhythm control mechanisms, offering precise and reliable data for studying physiological and pathological conditions.
Contact us now!
We are committed to making your research easier, more accurate, and more efficient and helping you build confidence in your data! We have provided services for a large number of customers, giving us rich experiences in offering customized, professional solutions according to your needs.