#Industry News
The Tow-Int Tech Animal Metabolism Monitoring System
Supports Research on Anorexia and Obesity
Introduction
Recently, domestic researchers, including Zijun Chen, Yixiao Luo, Yingjie Zhu, and others, studied the mechanism of action of liraglutide in mice. They discovered that GLP-1 receptor (GLP-1R) positive neurons in the lateral septum (LS) play a crucial role in the anorexic and weight-loss effects of liraglutide. Their findings were published in The Journal of Clinical Investigation, which had an impact factor of 15.9 in 2023 and a five-year impact factor of 14.6.
1. Research Background
Obesity is a global health threat, and liraglutide, a glucagon-like peptide-1 (GLP-1) analog, is used to treat obesity. However, the specific neuronal sites of its action remain unclear. GLP-1R-expressing cells in the central nervous system are critical for the anorexic and weight-loss effects of liraglutide. The hypothalamus has been a major research focus, but genetic deletion of GLP-1R in this region does not affect liraglutide’s action. The hindbrain regions, such as the caudal area, have also been of interest, though contradictory findings exist. The lateral septum (LS) also expresses GLP-1R and is involved in energy regulation, but the physiological function of its neurons and their role in liraglutide’s effects remain undefined.
2. Research Objective
The study aims to reveal the role of GLP-1Rs in the lateral septum in the physiological and pharmacological effects of liraglutide, including the regulation of energy homeostasis and their involvement in the anorexic and weight-loss effects induced by liraglutide.
3. Experimental Methods
Animal Models: Various transgenic mice were used, including GLP-1R-ires-Cre mice, Ai14 reporter mice, Rosa26-LSL-Cas9 mice, and wild-type C57BL/6J mice.
Techniques:
Immunohistochemistry: To determine the distribution of GLP-1Rs in the mouse brain.
Viral Vector Injection: Including the use of CRISPR/Cas9 technology to selectively knock out GLP-1R expression in specific neurons or overexpress GLP-1R.
Chemogenetics and Optogenetics: To activate or inhibit GLP-1R positive neurons in the lateral septum.
Fiber Photometry: To record neuronal activity.
Electrophysiology: To study synaptic transmission in neurons.
Behavioral Experiments: Including food intake measurements, body weight assessments, oral glucose tolerance tests, and conditioned taste aversion tests.
4. Experimental Results
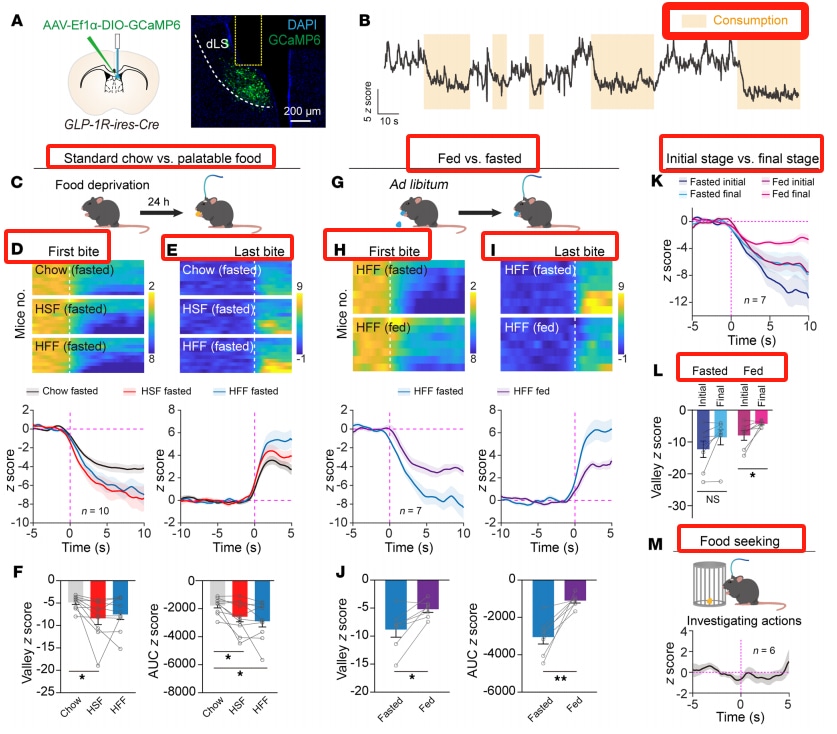
Liraglutide activates GLP-1R neurons in the lateral septum. Immunohistochemistry revealed that GLP-1R positive neurons are densely distributed in the dorsal part of the lateral septum (dLS). After intraperitoneal injection of liraglutide, c-Fos expression was elevated in the LS, paraventricular nucleus (PVN), and caudal area (AP). By crossing GLP-1R-ires-Cre mice with Ai14 reporter mice, GLP-1R positive neurons in the LS were labeled, and it was found that intraperitoneal injection of liraglutide significantly upregulated c-Fos expression in these neurons. Local infusion of liraglutide into the LS also had the same effect, increasing the amplitude of excitatory postsynaptic currents.
GLP-1R Knockout in the LS Weakens the Effects of Liraglutide
Using CRISPR/Cas9 technology, GLP-1R expression was specifically knocked out in GLP-1R positive neurons in the dorsal lateral septum (dLS). The knockout did not affect baseline food intake or body weight in the mice but weakened the effects of liraglutide on food intake and weight loss. Knocking out GLP-1R in the paraventricular nucleus (PVN) and arcuate nucleus (Arc) did not alter liraglutide's anorexic effects.
Overexpression of GLP-1R in the dLS Reduces Food Consumption in Satiety Mice
Overexpression of GLP-1R in the dorsal lateral septum (dLS) significantly reduced food consumption in satiety mice, but had no effect on food intake in fasting mice. Additionally, it did not alter chronic body weight or metabolic parameters.
Silencing LS GLP-1R Neurons Increases Food Consumption and Promotes Obesity
Silencing LS GLP-1R neurons (via injection of AAV expressing tetanus toxin) increases high-fat food intake and body weight, as well as the consumption of palatable foods. However, it does not affect glucose tolerance or water consumption, but it increases energy expenditure and the respiratory exchange ratio. Silencing these neurons weakens the effect of liraglutide on food intake and weight loss, but does not affect liraglutide-induced nausea.
Activity of LS GLP-1R Neurons Rapidly Decreases During Feeding
Fiber photometry recordings showed that, when food-deprived mice began to eat, the Ca²⁺ signal of LS GLP-1R neurons significantly decreased, returning to baseline levels after eating ceased. The decline was more pronounced when the food was more palatable. When high-fat food was presented to satiety mice, the inhibitory response was smaller, and the inhibition during feeding gradually diminished. Food-seeking behavior did not affect neuronal activity.
Activation of LS GLP-1R Neurons Reduces Food Consumption
Activation of LS GLP-1R neurons via chemogenetics or optogenetics can reduce food intake and suppress appetite without affecting glucose homeostasis. LS GLP-1R neurons project to several brain regions involved in eating regulation.
5. Research Conclusion
Liraglutide activates GLP-1R positive neurons in the lateral septum, and the GLP-1Rs in this region play an important role in liraglutide’s anorexic and weight-reducing effects. LS GLP-1R neurons are key in both physiological satiety and liraglutide-induced effects, providing new insights into the mechanism of liraglutide's action and potential targeted intervention points for obesity treatment.
Research Outcomes:
Deepening Understanding of Obesity Mechanisms: This study clarifies the importance of GLP-1R positive neurons in the lateral septum in regulating energy homeostasis, contributing to understanding the neurobiological mechanisms of human obesity. Obesity is closely related to imbalances in energy intake and expenditure, and the neural pathways revealed in this study play a critical role, offering a new perspective on the pathophysiology of obesity.
Explaining Drug Side Effect Mechanisms: The study found that LS GLP-1R neurons do not participate in liraglutide-induced nausea, suggesting that other brain regions are involved in the nausea response. This helps explain why some patients experience side effects like nausea when using GLP-1R agonists (e.g., liraglutide) to treat obesity or diabetes, providing a theoretical foundation for further research to address these side effects.
Identifying Potential Drug Targets: The research highlights the critical role of GLP-1Rs in the lateral septum in liraglutide’s effects, suggesting that this region or its related neurons may be potential targets for treating obesity and related metabolic diseases. Developing drugs targeting this pathway could improve the efficacy and safety of treatments while reducing side effects.
Optimizing Drug Treatment Strategies: The findings may help develop more precise drug treatment methods, such as modulating the activity of GLP-1R positive neurons in the lateral septum to enhance the anorexic and weight-loss effects of liraglutide while avoiding or reducing adverse effects like nausea. This opens up possibilities for personalized medicine.
Promoting Combined Treatment Approaches: Given that GLP-1Rs in different brain regions may work together to mediate the effects of liraglutide, future drug development could consider designing combined treatment strategies targeting multiple relevant pathways or brain regions to enhance the effectiveness of obesity treatments and related diseases.
This study used the Animal Metabolism Monitoring System from Tow-Int Tech, which measures parameters such as energy consumption, oxygen intake (VO₂), carbon dioxide output (VCO₂), respiratory exchange ratio, food and water intake, and other metabolic parameters. This system allows for long-term monitoring in mice under natural activity conditions, providing data support for studying the effects of drugs or neuronal modulation on energy metabolism.
Measuring Energy Expenditure (EE):
Energy expenditure is measured by monitoring the oxygen consumption (VO₂) and carbon dioxide output (VCO₂) of mice over a period of time in the energy metabolism system, and then calculating energy expenditure using specific formulas. This helps to understand the changes in energy consumption of mice under different experimental conditions (e.g., activation or inhibition of LS GLP-1R neurons) and assess the impact of these manipulations on the overall energy metabolism level of the mice.
Monitoring Oxygen Consumption (VO₂) and Carbon Dioxide Output (VCO₂):
Real-time and continuous measurements of VO₂ and VCO₂ in mice within the energy metabolism system provide gas exchange data under different activity states (such as active and resting periods). The study found that under certain experimental conditions (e.g., after silencing LS GLP-1R neurons), VO₂ and VCO₂ levels were altered. This provides critical data for analyzing the relationship between neuronal activity and energy metabolism.
Calculating Respiratory Exchange Ratio (RER):
The RER is the ratio of VCO₂ production to VO₂ consumption and reflects the metabolic utilization of carbohydrates and fats in the mouse. The study calculates the RER using data from the energy metabolism system and found that while VO₂ and VCO₂ changed under some conditions (e.g., after silencing LS GLP-1R neurons), the RER did not change. This suggests that the metabolic substrate utilization of the mice did not undergo substantial changes under these conditions, further deepening our understanding of the neuronal regulation of energy metabolism.
Simultaneous Measurement of Food and Water Intake:
The energy metabolism system can measure energy metabolism parameters while simultaneously recording the food and water intake of the mice. This allows for a comprehensive analysis of the balance between energy intake and expenditure, as well as the effects of different experimental treatments on this balance. For example, when studying the relationship between LS GLP-1R neuron manipulations and changes in food intake, combining data on energy consumption measured by the metabolic cage provides a more complete understanding of the role of these neurons in energy homeostasis regulation.
The Tow-Int Tech Animal Metabolism Monitoring System has provided comprehensive and continuous energy metabolism data for mice, which is crucial for revealing the mechanisms by which LS GLP-1R neurons regulate energy balance. It provides strong support for subsequent analysis and conclusions.
Contact us now!
We are committed to making your research easier, more accurate, and more efficient and helping you build confidence in your data! We have provided services for a large number of customers, giving us rich experiences in offering customized, professional solutions according to your needs.